Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30
- PMID: 20096356
- PMCID: PMC2868926
- DOI: 10.1016/j.nbd.2010.01.010
Dominant connexin26 mutants associated with human hearing loss have trans-dominant effects on connexin30
Abstract
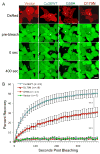
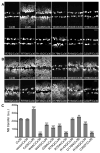
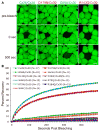
Dominant mutations in GJB2, the gene encoding the human gap junction protein connexin26 (Cx26), cause hearing loss. We investigated whether dominant Cx26 mutants interact directly with Cx30. HeLa cells stably expressing nine dominant Cx26 mutants, six associated with non-syndromic hearing loss (W44C, W44S, R143Q, D179N, R184Q and C202F) and three associated with hearing loss and palmoplantar keratoderma (G59A, R75Q and R75W), individually or together with Cx30, were analyzed by immunocytochemistry, co-immunoprecipitation, and functional assays (scrape-loading and/or fluorescence recovery after photobleaching). When expressed alone, all mutants formed gap junction plaques, but with impaired intercellular dye transfer. When expressed with Cx30, all mutants co-localized and co-immunoprecipitated with Cx30, indicating they likely co-assembled into heteromers. Furthermore, 8/9 Cx26 mutants inhibited the transfer of neurobiotin or calcein, indicating that these Cx26 mutants have trans-dominant effects on Cx30, an effect that may contribute to the pathogenesis of hearing loss.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures




References
-
- Ahmad S, et al. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. - PubMed
-
- Bakirtzis G, et al. Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum Mol Genet. 2003;12:1737–1744. - PubMed
-
- Beltramello M, et al. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun. 2003;305:1024–1033. - PubMed
-
- Beltramello M, et al. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

