Endocytosis is required for efficient apical constriction during Xenopus gastrulation
- PMID: 20096583
- PMCID: PMC3310928
- DOI: 10.1016/j.cub.2009.12.021
Endocytosis is required for efficient apical constriction during Xenopus gastrulation
Abstract
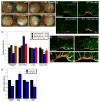
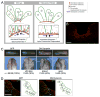
Coordinated apical constriction (AC) in epithelial sheets drives tissue invagination [1, 2] and is required for diverse morphogenetic movements such as gastrulation [3], neurulation [4, 5], and organogenesis [6]. We showed previously that actomyosin contractility drives AC in Xenopus laevis bottle cells [7]; however, it remained unclear whether it does so in concert with other processes. Here we report that endocytosis-driven membrane remodeling is required for efficient AC. We found endosomes exclusively in bottle cells in the early gastrula. Disrupting endocytosis with dominant-negative dynamin or rab5 perturbed AC, with a significant decrease in constriction rate late in the process, suggesting that endocytosis operates downstream of actomyosin contractility to remove excess membrane. Additionally, disrupting endocytosis during neurulation inhibits AC in hingepoint cells, resulting in neural tube closure defects. Thus, membrane remodeling during AC could be a general mechanism to achieve efficient invagination in embryos.
Figures


References
-
- Lewis W. Mechanics of Invagination. Anat Rec. 1947;97:139–156. - PubMed
-
- Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–462. - PubMed
-
- Kimberly EL, Hardin J. Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev Biol. 1998;204:235–250. - PubMed
-
- Burnside B. Microtubules and microfilaments in newt neuralation. Dev Biol. 1971;26:416–441. - PubMed
-
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

