Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation
- PMID: 20096584
- PMCID: PMC2990526
- DOI: 10.1016/j.cub.2009.11.072
Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation
Abstract
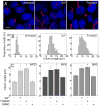
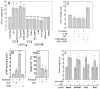
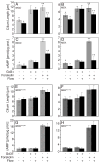
The primary cilium acts as a transducer of extracellular stimuli into intracellular signaling [1, 2]. Its regulation, particularly with respect to length, has been defined primarily by genetic experiments and human disease states in which molecular components that are necessary for its proper construction have been mutated or deleted [1]. However, dynamic modulation of cilium length, a phenomenon observed in ciliated protists [3, 4], has not been well-characterized in vertebrates. Here we demonstrate that decreased intracellular calcium (Ca(2+)) or increased cyclic AMP (cAMP), and subsequent protein kinase A activation, increases primary cilium length in mammalian epithelial and mesenchymal cells. Anterograde intraflagellar transport is sped up in lengthened cilia, potentially increasing delivery flux of cilium components. The cilium length response creates a negative feedback loop whereby fluid shear-mediated deflection of the primary cilium, which decreases intracellular cAMP, leads to cilium shortening and thus decreases mechanotransductive signaling. This adaptive response is blocked when the autosomal-dominant polycystic kidney disease (ADPKD) gene products, polycystin-1 or -2, are reduced. Dynamic regulation of cilium length is thus intertwined with cilium-mediated signaling and provides a natural braking mechanism in response to external stimuli that may be compromised in PKD.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells.PLoS One. 2014 Sep 22;9(9):e108470. doi: 10.1371/journal.pone.0108470. eCollection 2014. PLoS One. 2014. PMID: 25243405 Free PMC article.
-
An extracellular vesicle based hypothesis for the genesis of the polycystic kidney diseases.Extracell Vesicle. 2024 Dec;4:100048. doi: 10.1016/j.vesic.2024.100048. Epub 2024 Jul 26. Extracell Vesicle. 2024. PMID: 39886526 Free PMC article.
-
Genetic reduction of cilium length by targeting intraflagellar transport 88 protein impedes kidney and liver cyst formation in mouse models of autosomal polycystic kidney disease.Kidney Int. 2020 Nov;98(5):1225-1241. doi: 10.1016/j.kint.2020.05.049. Epub 2020 Jun 28. Kidney Int. 2020. PMID: 32610050
-
Factors that influence primary cilium length.Acta Med Okayama. 2011 Oct;65(5):279-85. doi: 10.18926/AMO/47009. Acta Med Okayama. 2011. PMID: 22037264 Review.
-
Role of primary cilia in the pathogenesis of polycystic kidney disease.J Am Soc Nephrol. 2007 May;18(5):1381-8. doi: 10.1681/ASN.2006111215. Epub 2007 Apr 11. J Am Soc Nephrol. 2007. PMID: 17429051 Review.
Cited by
-
Carcinogens induce loss of the primary cilium in human renal proximal tubular epithelial cells independently of effects on the cell cycle.Am J Physiol Renal Physiol. 2012 Apr 15;302(8):F905-16. doi: 10.1152/ajprenal.00427.2011. Epub 2012 Jan 18. Am J Physiol Renal Physiol. 2012. PMID: 22262483 Free PMC article.
-
Potential Therapeutic Targets for Olfactory Dysfunction in Ciliopathies Beyond Single-Gene Replacement.Chem Senses. 2021 Jan 1;46:bjab010. doi: 10.1093/chemse/bjab010. Chem Senses. 2021. PMID: 33690843 Free PMC article. Review.
-
The regulation of cilium assembly and disassembly in development and disease.Development. 2018 Sep 17;145(18):dev151407. doi: 10.1242/dev.151407. Development. 2018. PMID: 30224385 Free PMC article. Review.
-
Anomalies in Dopamine Transporter Expression and Primary Cilium Distribution in the Dorsal Striatum of a Mouse Model of Niemann-Pick C1 Disease.Front Cell Neurosci. 2019 May 24;13:226. doi: 10.3389/fncel.2019.00226. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31178699 Free PMC article.
-
Kinesin 1 regulates cilia length through an interaction with the Bardet-Biedl syndrome related protein CCDC28B.Sci Rep. 2018 Feb 14;8(1):3019. doi: 10.1038/s41598-018-21329-6. Sci Rep. 2018. PMID: 29445114 Free PMC article.
References
-
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. - PubMed
-
- Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol. 1993;265:F416–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

