Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists
- PMID: 20096719
- PMCID: PMC2858063
- DOI: 10.1016/j.steroids.2010.01.010
Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists
Abstract
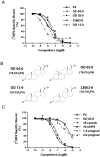
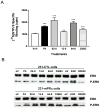
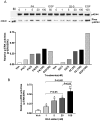
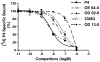
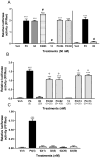
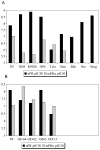
Recent results showing that the binding characteristics of 33 steroids for human membrane progesterone receptor alpha (hu-mPRalpha) differ from those for the nuclear progesterone receptor (nPR) suggest that hu-mPRalpha-specific agonists can be identified for investigating its physiological functions. The binding affinities of an additional 21 steroids for hu-mPRalpha were determined to explore the structure-activity relationships in more detail and to identify potent, specific mPRalpha agonists. Four synthetic progesterone derivatives with methyl or methylene groups on positions 18 or 19, 18a-methylprogesterone (18-CH(3)P4, Org OE 64-0), 13-ethenyl-18-norprogesterone (18-CH(2)P4, Org 33663-0), 19a-methylprogesterone (19-CH(3)P4, Org OD 13-0) and 10-ethenyl-19-norprogesterone (19-CH(2)P4, Org OD 02-0), showed similar or higher affinities than progesterone for hu-mPRalpha and displayed mPRalpha agonist activities in G-protein and MAP kinase activation assays. All four steroids also bound to the nPR in cytosolic fractions of MCF-7 cells. However, two compounds, 19-CH(2)P4 and 19-CH(3)P4, showed no nPR agonist activity in a nPR reporter assay and therefore are selective mPRalpha agonists suitable for physiological investigations. The structure-binding relationships of the combined series of 54 steroids for hu-mPRalpha deviated strikingly from those of a published set of 60 3-keto or 3-desoxy steroids for nPR. Close correlations were observed between the receptor binding affinities of the steroids and their physicochemical properties calculated by comparative molecular field analysis (CoMFA) for both hu-mPRalpha and nPR. A comparison of the CoMFA field graphs for the two receptors revealed several differences in the structural features required for binding to hu-mPRalpha and nPR which could be exploited to develop additional mPR-specific ligands.
Figures


Similar articles
-
Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors.Endocrinology. 2014 Mar;155(3):1107-19. doi: 10.1210/en.2013-1991. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424068 Free PMC article.
-
Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway.Breast Cancer Res. 2010;12(3):R34. doi: 10.1186/bcr2588. Epub 2010 Jun 11. Breast Cancer Res. 2010. PMID: 20540763 Free PMC article.
-
Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins.Endocrinology. 2007 Feb;148(2):705-18. doi: 10.1210/en.2006-0974. Epub 2006 Nov 2. Endocrinology. 2007. PMID: 17082257
-
Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics.Cells. 2022 May 30;11(11):1785. doi: 10.3390/cells11111785. Cells. 2022. PMID: 35681480 Free PMC article. Review.
-
Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): Identification of mPRalpha on human sperm and its association with sperm motility.Steroids. 2009 Jul;74(7):614-21. doi: 10.1016/j.steroids.2008.10.020. Epub 2008 Nov 21. Steroids. 2009. PMID: 19071147 Review.
Cited by
-
Twenty years of transcriptomics, 17alpha-ethinylestradiol, and fish.Gen Comp Endocrinol. 2020 Jan 15;286:113325. doi: 10.1016/j.ygcen.2019.113325. Epub 2019 Nov 13. Gen Comp Endocrinol. 2020. PMID: 31733209 Free PMC article. Review.
-
Establishment of a graphene quantum dot (GQD) based steroid binding assay for the nuclear progesterone receptor (pgr).Biochem Biophys Rep. 2024 Mar 25;38:101691. doi: 10.1016/j.bbrep.2024.101691. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38571552 Free PMC article.
-
Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors.Horm Cancer. 2012 Jun;3(3):101-12. doi: 10.1007/s12672-012-0106-x. Horm Cancer. 2012. PMID: 22350867 Free PMC article.
-
Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: The role of membrane progesterone receptors.Gene. 2016 Oct 10;591(1):6-13. doi: 10.1016/j.gene.2016.06.044. Epub 2016 Jun 24. Gene. 2016. PMID: 27349565 Free PMC article.
-
Progesterone exerts a neuroprotective action in a Parkinson's disease human cell model through membrane progesterone receptor α (mPRα/PAQR7).Front Endocrinol (Lausanne). 2023 Mar 10;14:1125962. doi: 10.3389/fendo.2023.1125962. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967764 Free PMC article.
References
-
- Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor site. Steroids. 1997;62:268–303. - PubMed
-
- Cramer RD, III, Patterson DE, Bunce JD. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988;110:5959–67. - PubMed
-
- Xing L, Welsh WJ, Tong W, Perkins R, Sheehan DM. Comparison of estrogen receptor alpha and beta subtypes based on comparative molecular field analysis (CoMFA) SAR QSAR Environ Res. 1999;10:215–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

