Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies
- PMID: 20096780
- PMCID: PMC2862783
- DOI: 10.1016/j.matbio.2010.01.005
Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies
Abstract
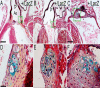
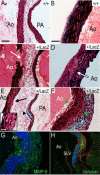
Here, we demonstrate that ADAMTS9, a highly conserved versican-degrading protease, is required for correct cardiovascular development and adult homeostasis. Analysis of Adamts9(+/LacZ) adult mice revealed anomalies in the aortic wall, valvulosinus and valve leaflets. Abnormal myocardial projections and 'spongy' myocardium consistent with non-compaction of the left ventricle were also found in Adamts9(+/LacZ) mice. During development, Adamts9 was expressed in derivatives of the Secondary Heart Field, vascular smooth muscle cells in the arterial wall, mesenchymal cells of the valves, and non-myocardial cells of the ventricles, but expression also continued in the adult heart and ascending aorta. Thus, the adult cardiovascular anomalies found in Adamts9(+/LacZ) hearts could result from subtle developmental alterations in extracellular matrix remodeling or defects in adult homeostasis. The valvular and aortic anomalies of Adamts9(+/LacZ) hearts were associated with accumulation of versican and a decrease in cleaved versican relative to WT littermates. These data suggest a potentially important role for ADAMTS9 cleavage of versican, or other, as yet undefined substrates in development and allostasis of cardiovascular extracellular matrix. In addition, these studies identify ADAMTS9 as a potential candidate gene for congenital cardiac anomalies. Mouse models of ADAMTS9 deficiency may be useful to study myxomatous valve degeneration.
Copyright 2010 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures






Similar articles
-
The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis.Matrix Biol. 2014 Apr;35:34-41. doi: 10.1016/j.matbio.2014.01.005. Epub 2014 Jan 18. Matrix Biol. 2014. PMID: 24444773 Free PMC article. Review.
-
Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation.Development. 2010 Dec;137(23):4029-38. doi: 10.1242/dev.050591. Epub 2010 Nov 1. Development. 2010. PMID: 21041365 Free PMC article.
-
Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease.Dev Biol. 2011 Sep 1;357(1):152-64. doi: 10.1016/j.ydbio.2011.06.041. Epub 2011 Jul 1. Dev Biol. 2011. PMID: 21749862 Free PMC article.
-
Maladaptive matrix remodeling and regional biomechanical dysfunction in a mouse model of aortic valve disease.Matrix Biol. 2012 Apr;31(3):197-205. doi: 10.1016/j.matbio.2012.01.001. Epub 2012 Jan 12. Matrix Biol. 2012. PMID: 22265892 Free PMC article.
-
Structure, function and regulation of versican: the most abundant type of proteoglycan in the extracellular matrix.Acta Med Iran. 2013;51(11):740-50. Acta Med Iran. 2013. PMID: 24390942 Review.
Cited by
-
The evolutionary conservation of the A Disintegrin-like and Metalloproteinase domain with Thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish.BMC Evol Biol. 2015 Feb 15;15:22. doi: 10.1186/s12862-015-0281-9. BMC Evol Biol. 2015. PMID: 25879701 Free PMC article.
-
Development of myotendinous-like junctions that anchor cardiac valves requires fibromodulin and lumican.Dev Dyn. 2016 Oct;245(10):1029-42. doi: 10.1002/dvdy.24435. Epub 2016 Aug 25. Dev Dyn. 2016. PMID: 27503167 Free PMC article.
-
Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction.J Biol Chem. 2013 Jan 4;288(1):520-8. doi: 10.1074/jbc.M112.393629. Epub 2012 Nov 21. J Biol Chem. 2013. PMID: 23172227 Free PMC article.
-
Adamts5-/- Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):2067-2081. doi: 10.1161/ATVBAHA.119.313077. Epub 2019 Aug 1. Arterioscler Thromb Vasc Biol. 2019. PMID: 31366218 Free PMC article.
-
Zinc transporter Slc39a8 is essential for cardiac ventricular compaction.J Clin Invest. 2018 Feb 1;128(2):826-833. doi: 10.1172/JCI96993. Epub 2018 Jan 16. J Clin Invest. 2018. PMID: 29337306 Free PMC article.
References
-
- Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–5. - PubMed
-
- Aspberg A, Adam S, Kostka G, Timpl R, Heinegard D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. Journal of Biological Chemistry. 1999;274:20444–9. - PubMed
-
- Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008;294:H1197–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR53890/AR/NIAMS NIH HHS/United States
- R01 HL033756/HL/NHLBI NIH HHS/United States
- R01 AR049930/AR/NIAMS NIH HHS/United States
- R01 HL084285/HL/NHLBI NIH HHS/United States
- RR0164-34/RR/NCRR NIH HHS/United States
- HL084285/HL/NHLBI NIH HHS/United States
- R01 HL095067/HL/NHLBI NIH HHS/United States
- AR49930/AR/NIAMS NIH HHS/United States
- R01 AR053890/AR/NIAMS NIH HHS/United States
- HL095696/HL/NHLBI NIH HHS/United States
- P20 RR016434/RR/NCRR NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- R56 AR049930/AR/NIAMS NIH HHS/United States
- HL095067/HL/NHLBI NIH HHS/United States
- R01 HL095696/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

