Critical role of cholesterol in bovine herpesvirus type 1 infection of MDBK cells
- PMID: 20097021
- PMCID: PMC7117431
- DOI: 10.1016/j.vetmic.2009.12.031
Critical role of cholesterol in bovine herpesvirus type 1 infection of MDBK cells
Abstract
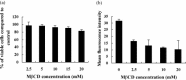
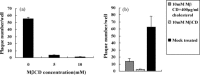
Cholesterol is involved in the life cycle of many viruses. Here, we examined the role of cholesterol for both viral envelope and target cell membrane for bovine herpesvirus type 1 (BoHV-1) infection. Cholesterol depletion by pretreatment of Madin-Darby bovine kidney (MDBK) cells with a cholesterol-sequestering drug methyl-beta-cyclodextrin (MbetaCD), inhibited the production of BoHV-1 in a dose-dependent manner. This inhibitory effect was partially reversed by cholesterol replenishment, indicating that the reduction was caused by cholesterol depletion. Cholesterol depletion at the post-entry stage only had a mild effect on the virus production. However, cell membrane cholesterol depletion did not reduce the virus attachment. In addition, treatment of BoHV-1 particles with MbetaCD also reduced the virus infectivity significantly and the effect was partially reversed by addition of exogenous cholesterol. Taken together, these data implicated that cell membrane cholesterol mainly contributed to BoHV-1 entry into MDBK cells and the viral envelope cholesterol was also essential for the virus infectivity.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Critical role of the lipid rafts in caprine herpesvirus type 1 infection in vitro.Virus Res. 2016 Jan 4;211:186-93. doi: 10.1016/j.virusres.2015.10.013. Epub 2015 Oct 22. Virus Res. 2016. PMID: 26475997 Free PMC article.
-
Bovine Herpesvirus 1 Entry by a Low-pH Endosomal Pathway.J Virol. 2018 Sep 26;92(20):e00839-18. doi: 10.1128/JVI.00839-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045989 Free PMC article.
-
Bovine single chain Fv antibody inhibits bovine herpesvirus-1 infectivity by targeting viral glycoprotein D.Appl Microbiol Biotechnol. 2017 Dec;101(23-24):8331-8344. doi: 10.1007/s00253-017-8566-0. Epub 2017 Oct 23. Appl Microbiol Biotechnol. 2017. PMID: 29063173
-
Biology of bovine herpesvirus 5.Vet J. 2010 May;184(2):138-45. doi: 10.1016/j.tvjl.2009.03.035. Epub 2009 May 5. Vet J. 2010. PMID: 19409823 Review.
-
Distinctive features of bovine alphaherpesvirus types 1 and 5 and the virus-host interactions that might influence clinical outcomes.Arch Virol. 2020 Feb;165(2):285-301. doi: 10.1007/s00705-019-04494-5. Epub 2019 Dec 16. Arch Virol. 2020. PMID: 31845150 Review.
Cited by
-
Critical role of the lipid rafts in caprine herpesvirus type 1 infection in vitro.Virus Res. 2016 Jan 4;211:186-93. doi: 10.1016/j.virusres.2015.10.013. Epub 2015 Oct 22. Virus Res. 2016. PMID: 26475997 Free PMC article.
-
Induction of the Unfolded Protein Response during Bovine Alphaherpesvirus 1 Infection.Viruses. 2020 Sep 2;12(9):974. doi: 10.3390/v12090974. Viruses. 2020. PMID: 32887282 Free PMC article.
-
Rotavirus C Replication in Porcine Intestinal Enteroids Reveals Roles for Cellular Cholesterol and Sialic Acids.Viruses. 2022 Aug 20;14(8):1825. doi: 10.3390/v14081825. Viruses. 2022. PMID: 36016447 Free PMC article.
-
Role of the lipid rafts in the life cycle of canine coronavirus.J Gen Virol. 2015 Feb;96(Pt 2):331-337. doi: 10.1099/vir.0.070870-0. Epub 2014 Nov 7. J Gen Virol. 2015. PMID: 25381058 Free PMC article.
-
Curcumin as an Antiviral Agent.Viruses. 2020 Oct 31;12(11):1242. doi: 10.3390/v12111242. Viruses. 2020. PMID: 33142686 Free PMC article. Review.
References
-
- Brown D.A., London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

