Genetic linkage of autologous T cell epitopes in a chimeric recombinant construct improves anti-parasite and anti-disease protective effect of a malaria vaccine candidate
- PMID: 20097151
- PMCID: PMC2844075
- DOI: 10.1016/j.vaccine.2010.01.019
Genetic linkage of autologous T cell epitopes in a chimeric recombinant construct improves anti-parasite and anti-disease protective effect of a malaria vaccine candidate
Abstract
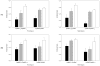
We have reported the design of polyvalent synthetic and recombinant chimeras that include promiscuous T cell epitopes as a viable delivery system for pre-erythrocytic subunit malaria vaccines. To further assess the ability of several Plasmodium T cell epitopes to enhance vaccine potency, we designed a synthetic gene encoding four Plasmodium yoelii merozoite surface protein 1 (PyMSP1) CD4(+) promiscuous T cell epitopes fused in tandem to the homologous carboxyl terminal PyMSP1(19) fragment. This Recombinant Modular Chimera (PyRMC-MSP1(19)) was tested for immunogenicity and protective efficacy in comparative experiments with a recombinant protein expressing only the PyMSP1(19) fragment. Both proteins induced comparable antibody responses. However PyRMC-MSP1(19) elicited higher anti-parasite antibody titers and more robust protection against both hyper-parasitemia and malarial anemia. Most importantly, passive transfer of anti-PyRMC-MSP1(19), but not anti-PyMSP1(19) antibodies protected against heterologous challenge. These studies show that protective efficacy can be significantly improved by inclusion of an array of autologous promiscuous T cell epitopes in vaccine constructs.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Organization WH, editor. WHO. World malaria report 2008. Geneva: WHO Press; 2008.
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9(7):725–32. - PubMed
-
- Conway DJ, Cavanagh DR, Tanabe K, Roper C, Mikes ZS, Sakihama N, et al. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med. 2000;6(6):689–92. - PubMed
-
- Kester KE, Cummings JF, Ockenhouse CF, Nielsen R, Hall BT, Gordon DM, et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2008;26(18):2191–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

