Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys
- PMID: 20097152
- PMCID: PMC2837772
- DOI: 10.1016/j.vaccine.2010.01.022
Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys
Abstract
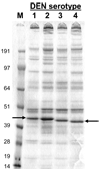
Truncated recombinant dengue virus envelope protein subunits (80E) are efficiently expressed using the Drosophila Schneider-2 (S2) cell expression system. Binding of conformationally sensitive antibodies as well as X-ray crystal structural studies indicate that the recombinant 80E subunits are properly folded native-like proteins. Combining the 80E subunits from each of the four dengue serotypes with ISCOMATRIX adjuvant, an adjuvant selected from a set of adjuvants tested for maximal and long lasting immune responses, results in high titer virus neutralizing antibody responses. Immunization of mice with a mixture of all four 80E subunits and ISCOMATRIX adjuvant resulted in potent virus neutralizing antibody responses to each of the four serotypes. The responses to the components of the tetravalent mixture were equivalent to the responses to each of the subunits administered individually. In an effort to evaluate the potential protective efficacy of the Drosophila expressed 80E, the dengue serotype 2 (DEN2-80E) subunit was tested in both the mouse and monkey challenge models. In both models protection against viral challenge was achieved with low doses of antigen in the vaccine formulation. In non-human primates, low doses of the tetravalent formulation induced good virus neutralizing antibody titers to all four serotypes and protection against challenge with the two dengue virus serotypes tested. In contrast to previous reports, where subunit vaccine candidates have generally failed to induce potent, protective responses, native-like soluble 80E proteins expressed in the Drosophila S2 cells and administered with appropriate adjuvants are highly immunogenic and capable of eliciting protective responses in both mice and monkeys. These results support the development of a dengue virus tetravalent vaccine based on the four 80E subunits produced in the Drosophila S2 cell expression system.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Halstead SB. Pathogenesis of Dengue: Challenges to Molecular Biology. Science. 1988;239:476–481. - PubMed
-
- Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. - PubMed
-
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Research. 2002;33:330–342. - PubMed
-
- Rigau-Perez JG, Gubler DJ, Vorndam AV, Clark GG. Dengue surveillance- United States, 1986–1992. MMWR. 1994;43:7–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

