The protein kingdom extended: ordered and intrinsically disordered proteins, their folding, supramolecular complex formation, and aggregation
- PMID: 20097220
- PMCID: PMC2916636
- DOI: 10.1016/j.pbiomolbio.2010.01.003
The protein kingdom extended: ordered and intrinsically disordered proteins, their folding, supramolecular complex formation, and aggregation
Abstract
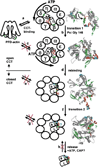
The native state of a protein is usually associated with a compact globular conformation possessing a rigid and highly ordered structure. At the turn of the last century certain studies arose which concluded that many proteins cannot, in principle, form a rigid globular structure in an aqueous environment, but they are still able to fulfill their specific functions--i.e., they are native. The existence of the disordered regions allows these proteins to interact with their numerous binding partners. Such interactions are often accompanied by the formation of complexes that possess a more ordered structure than the original components. The functional diversity of these proteins, combined with the variability of signals related to the various intra- and intercellular processes handled by these proteins and their capability to produce multi-variant and multi-directional responses allow them to form a unique regulatory net in a cell. The abundance of disordered proteins inside the cell is precisely controlled at the synthesis and clearance levels as well as via interaction with specific binding partners and post-translational modifications. Another recently recognized biologically active state of proteins is the functional amyloid. The formation of such functional amyloids is tightly controlled and therefore differs from the uncontrolled formation of pathogenic amyloids which are associated with the pathogenesis of several conformational diseases, the development of which is likely to be determined by the failures of the cellular regulatory systems rather than by the formation of the proteinaceous deposits and/or by the protofibril toxicity.
2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Aigle M, Lacroute F. Genetical aspects of [URE3], a non-mitochondrial, cytoplasmically inherited mutation in yeast. Mol Gen Genet. 1975;136:327–335. - PubMed
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Bader MW, Bardwell JC. Catalysis of disulfide bond formation and isomerization in Escherichia coli. Adv Protein Chem. 2001;59:283–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

