Modulation of type I IFN induction by a virulence determinant within the alphavirus nsP1 protein
- PMID: 20097400
- PMCID: PMC2830325
- DOI: 10.1016/j.virol.2009.12.031
Modulation of type I IFN induction by a virulence determinant within the alphavirus nsP1 protein
Abstract
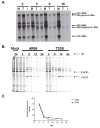
Alphaviruses are mosquito-borne viruses that cause serious human and animal diseases. Previous studies demonstrated that a determinant within the nsP1/nsP2 cleavage domain of the virulent Sindbis AR86 virus played a key role in regulating adult mouse virulence without adversely affecting viral replication. Additional characterization of this determinant demonstrated that a virus with the attenuating mutation induced more type I IFN production both in vivo and in vitro. Interestingly, this phenotype was not specific to the Sindbis AR86 virus, as a similar mutation in a distantly related alphavirus, Ross River Virus (RRV), also led to enhanced IFN induction. This effect was independent of virus-induced host shutoff, since IRF-3 phosphorylation, which occurs independently of de novo host transcription/translation, was induced more robustly in cells infected with the mutant viruses. Altogether, these results demonstrate that critical determinants within the nsP1/nsP2 cleavage domain play an important role in regulating alphavirus-induced IFN responses.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Decreased Virulence of Ross River Virus Harboring a Mutation in the First Cleavage Site of Nonstructural Polyprotein Is Caused by a Novel Mechanism Leading to Increased Production of Interferon-Inducing RNAs.mBio. 2018 Aug 21;9(4):e00044-18. doi: 10.1128/mBio.00044-18. mBio. 2018. PMID: 30131356 Free PMC article.
-
Identification of Natural Molecular Determinants of Ross River Virus Type I Interferon Modulation.J Virol. 2020 Mar 31;94(8):e01788-19. doi: 10.1128/JVI.01788-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996431 Free PMC article.
-
Attenuating mutations in nsP1 reveal tissue-specific mechanisms for control of Ross River virus infection.J Virol. 2014 Apr;88(7):3719-32. doi: 10.1128/JVI.02609-13. Epub 2014 Jan 15. J Virol. 2014. PMID: 24429363 Free PMC article.
-
Alphavirus Infection: Host Cell Shut-Off and Inhibition of Antiviral Responses.Viruses. 2016 Jun 11;8(6):166. doi: 10.3390/v8060166. Viruses. 2016. PMID: 27294951 Free PMC article. Review.
-
Molecular determinants of alphavirus neuropathogenesis in mice.J Gen Virol. 2016 Jun;97(6):1283-1296. doi: 10.1099/jgv.0.000467. Epub 2016 Mar 30. J Gen Virol. 2016. PMID: 27028153 Review.
Cited by
-
Early events in alphavirus replication determine the outcome of infection.J Virol. 2012 May;86(9):5055-66. doi: 10.1128/JVI.07223-11. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345447 Free PMC article.
-
Disruption of the Opal Stop Codon Attenuates Chikungunya Virus-Induced Arthritis and Pathology.mBio. 2017 Nov 14;8(6):e01456-17. doi: 10.1128/mBio.01456-17. mBio. 2017. PMID: 29138302 Free PMC article.
-
GETV nsP2 plays a critical role in the interferon antagonism and viral pathogenesis.Cell Commun Signal. 2023 Dec 18;21(1):361. doi: 10.1186/s12964-023-01392-x. Cell Commun Signal. 2023. PMID: 38110975 Free PMC article.
-
Differences in Processing Determinants of Nonstructural Polyprotein and in the Sequence of Nonstructural Protein 3 Affect Neurovirulence of Semliki Forest Virus.J Virol. 2015 Nov;89(21):11030-45. doi: 10.1128/JVI.01186-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311875 Free PMC article.
-
The role of innate versus adaptive immune responses in a mouse model of O'nyong-nyong virus infection.Am J Trop Med Hyg. 2013 Jun;88(6):1170-9. doi: 10.4269/ajtmh.12-0674. Epub 2013 Apr 8. Am J Trop Med Hyg. 2013. PMID: 23568285 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

