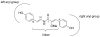Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter
- PMID: 20097565
- PMCID: PMC2848298
- DOI: 10.1016/j.bmcl.2010.01.016
Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter
Abstract
In previous work, botryllamides discovered from the marine ascidian Botryllus tyreus were characterized as selective inhibitors of the ABCG2 multidrug transporter. However, the structural basis for this activity could not be established. In this study, botryllamide F, the core botryllamide structure, and botryllamide G, the most potent botryllamide ABCG2 inhibitor, were synthesized along with a series of structural variants for evaluation of structure-activity relationships. The biological activity of synthetic botryllamide analogs implied that the 2-methoxy-p-coumaric acid portion, and the degree of double bond conjugation within this group, were critical for inhibition of ABCG2. However, variations in the substituents on the two aryl groups did not appear to significantly impact the potency or degree of inhibition.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Botryllamide G is an ABCG2 inhibitor that improves lapatinib delivery in mouse brain.Cancer Biol Ther. 2020;21(3):223-230. doi: 10.1080/15384047.2019.1683324. Epub 2019 Nov 10. Cancer Biol Ther. 2020. PMID: 31709896 Free PMC article.
-
Botryllamides: natural product inhibitors of ABCG2.ACS Chem Biol. 2009 Aug 21;4(8):637-47. doi: 10.1021/cb900134c. ACS Chem Biol. 2009. PMID: 19555120 Free PMC article.
-
Phenolic indeno[1,2-b]indoles as ABCG2-selective potent and non-toxic inhibitors stimulating basal ATPase activity.Drug Des Devel Ther. 2015 Jul 3;9:3481-95. doi: 10.2147/DDDT.S84982. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26170632 Free PMC article.
-
Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2).Anticancer Drugs. 2006 Mar;17(3):239-43. doi: 10.1097/00001813-200603000-00001. Anticancer Drugs. 2006. PMID: 16520651 Review.
-
Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2).AAPS J. 2009 Sep;11(3):541-52. doi: 10.1208/s12248-009-9132-1. Epub 2009 Jul 24. AAPS J. 2009. PMID: 19629710 Free PMC article. Review.
Cited by
-
Marine Natural Products as Models to Circumvent Multidrug Resistance.Molecules. 2016 Jul 8;21(7):892. doi: 10.3390/molecules21070892. Molecules. 2016. PMID: 27399665 Free PMC article. Review.
-
Ascidian Toxins with Potential for Drug Development.Mar Drugs. 2018 May 13;16(5):162. doi: 10.3390/md16050162. Mar Drugs. 2018. PMID: 29757250 Free PMC article. Review.
-
Marine natural products as breast cancer resistance protein inhibitors.Mar Drugs. 2015 Apr 3;13(4):2010-29. doi: 10.3390/md13042010. Mar Drugs. 2015. PMID: 25854646 Free PMC article. Review.
-
Creating and screening natural product libraries.Nat Prod Rep. 2020 Jul 1;37(7):893-918. doi: 10.1039/c9np00068b. Epub 2020 Mar 18. Nat Prod Rep. 2020. PMID: 32186299 Free PMC article. Review.
-
Bioactive dehydrotyrosyl and dehydrodopyl compounds of marine origin.Mar Drugs. 2010 Dec 6;8(12):2906-35. doi: 10.3390/md8122906. Mar Drugs. 2010. PMID: 21339956 Free PMC article. Review.
References
-
- Dean M, Annilo T. Annu Rev Genom Human Genet. 2005;6:123. - PubMed
-
- Robey RW, Polgar O, Deeken J, To KW, Bates SE. Cancer Metastasis Rev. 2007;26:39. - PubMed
-
- Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. Curr Drug Deliv. 2007;4:324. - PubMed
-
- Henrich CJ, Bokesch HR, Dean M, Bates SE, Robey R, Goncharova EI, Wilson JA, McMahon JB. J Biomol Screen. 2006;11:176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information