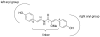Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter
- PMID: 20097565
- PMCID: PMC2848298
- DOI: 10.1016/j.bmcl.2010.01.016
Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter
Abstract
In previous work, botryllamides discovered from the marine ascidian Botryllus tyreus were characterized as selective inhibitors of the ABCG2 multidrug transporter. However, the structural basis for this activity could not be established. In this study, botryllamide F, the core botryllamide structure, and botryllamide G, the most potent botryllamide ABCG2 inhibitor, were synthesized along with a series of structural variants for evaluation of structure-activity relationships. The biological activity of synthetic botryllamide analogs implied that the 2-methoxy-p-coumaric acid portion, and the degree of double bond conjugation within this group, were critical for inhibition of ABCG2. However, variations in the substituents on the two aryl groups did not appear to significantly impact the potency or degree of inhibition.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Dean M, Annilo T. Annu Rev Genom Human Genet. 2005;6:123. - PubMed
-
- Robey RW, Polgar O, Deeken J, To KW, Bates SE. Cancer Metastasis Rev. 2007;26:39. - PubMed
-
- Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. Curr Drug Deliv. 2007;4:324. - PubMed
-
- Henrich CJ, Bokesch HR, Dean M, Bates SE, Robey R, Goncharova EI, Wilson JA, McMahon JB. J Biomol Screen. 2006;11:176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information