A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry
- PMID: 20097626
- PMCID: PMC2975562
- DOI: 10.1016/j.jchromb.2009.12.018
A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry
Abstract
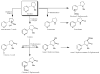
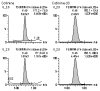
A novel validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) procedure was developed and fully validated for the simultaneous determination of nicotine-N-beta-D-glucuronide, cotinine-N-oxide, trans-3-hydroxycotinine, norcotinine, trans-nicotine-1'-oxide, cotinine, nornicotine, nicotine, anatabine, anabasine and cotinine-N-beta-D-glucuronide in human plasma or urine. Target analytes and corresponding deuterated internal standards were extracted by solid-phase extraction and analyzed by LC-MS/MS with electrospray ionization (ESI) using multiple reaction monitoring (MRM) data acquisition. Calibration curves were linear over the selected concentration ranges for each analyte, with calculated coefficients of determination (R(2)) of greater than 0.99. The total extraction recovery (%) was concentration dependent and ranged between 52-88% in plasma and 51-118% in urine. The limits of quantification for all analytes in plasma and urine were 1.0 ng/mL and 2.5 ng/mL, respectively, with the exception of cotinine-N-beta-D-glucuronide, which was 50 ng/mL. Intra-day and inter-day imprecision were < or = 14% and < or = 17%, respectively. Matrix effect (%) was sufficiently minimized to < or = 19% for both matrices using the described sample preparation and extraction methods. The target analytes were stable in both matrices for at least 3 freeze-thaw cycles, 24 h at room temperature, 24 h in the refrigerator (4 degrees C) and 1 week in the freezer (-20 degrees C). Reconstituted plasma and urine extracts were stable for at least 72 h storage in the liquid chromatography autosampler at 4 degrees C. The plasma procedure has been successfully applied in the quantitative determination of selected analytes in samples collected from nicotine-abstinent human participants as part of a pharmacokinetic study investigating biomarkers of nicotine use in plasma following controlled low dose (7 mg) transdermal nicotine delivery. Nicotine, cotinine, trans-3-hydroxycotinine and trans-nicotine-1'-oxide were detected in the particular sample presented herein. The urine procedure has been used to facilitate the monitoring of unauthorized tobacco use by clinical study participants at the time of physical examination (before enrollment) and on the pharmacokinetic study day.
2009 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Nov 15;879(30):3465-74. doi: 10.1016/j.jchromb.2011.09.026. Epub 2011 Sep 18. J Chromatogr B Analyt Technol Biomed Life Sci. 2011. PMID: 21963483 Free PMC article.
-
Determination of nicotine and nicotine metabolites in urine by hydrophilic interaction chromatography-tandem mass spectrometry: Potential use of smokeless tobacco products by ice hockey players.J Chromatogr A. 2010 Nov 26;1217(48):7528-38. doi: 10.1016/j.chroma.2010.10.005. Epub 2010 Oct 25. J Chromatogr A. 2010. PMID: 20980010
-
Quantitation of nicotine, its metabolites, and other related alkaloids in urine, serum, and plasma using LC-MS-MS.Methods Mol Biol. 2010;603:389-98. doi: 10.1007/978-1-60761-459-3_38. Methods Mol Biol. 2010. PMID: 20077091
-
Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry.J Pharm Biomed Anal. 2009 Jan 15;49(1):108-14. doi: 10.1016/j.jpba.2008.09.044. Epub 2008 Oct 8. J Pharm Biomed Anal. 2009. PMID: 19004590
-
A validated method for the determination of nicotine, cotinine, trans-3'-hydroxycotinine, and norcotinine in human plasma using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry.J Mass Spectrom. 2006 Jun;41(6):815-21. doi: 10.1002/jms.1039. J Mass Spectrom. 2006. PMID: 16705667
Cited by
-
Recent Developments in the Determination of Biomarkers of Tobacco Smoke Exposure in Biological Specimens: A Review.Int J Environ Res Public Health. 2021 Feb 11;18(4):1768. doi: 10.3390/ijerph18041768. Int J Environ Res Public Health. 2021. PMID: 33670326 Free PMC article. Review.
-
Recent Advances in Mass Spectrometry for the Identification of Neuro-chemicals and their Metabolites in Biofluids.Curr Neuropharmacol. 2013 Jul;11(4):436-64. doi: 10.2174/1570159X11311040007. Curr Neuropharmacol. 2013. PMID: 24381533 Free PMC article.
-
A Comparison of Direct and Indirect Analytical Approaches to Measuring Total Nicotine Equivalents in Urine.Cancer Epidemiol Biomarkers Prev. 2018 Aug;27(8):882-891. doi: 10.1158/1055-9965.EPI-18-0018. Epub 2018 May 31. Cancer Epidemiol Biomarkers Prev. 2018. PMID: 29853480 Free PMC article.
-
Quantitation of Urine Nicotine, Cotinine, and 3-OH-Cotinine by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS).Methods Mol Biol. 2024;2737:337-345. doi: 10.1007/978-1-0716-3541-4_31. Methods Mol Biol. 2024. PMID: 38036835
-
Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers' urine.PLoS One. 2014 Jul 11;9(7):e101816. doi: 10.1371/journal.pone.0101816. eCollection 2014. PLoS One. 2014. PMID: 25013964 Free PMC article.
References
-
- US Center for Disease Control and Prevention. Morbidity and Mortality Weekly Report. 2008;57(45):1226. - PubMed
-
- US Environmental Protection Agency. EPA/600/6-90/006F. 1992
-
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434. Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings.
-
- Clarke PBS, Quik M, Adlkofer F, Thurau K, editors. Effects of Nicotine on Biological Systems II. Birkhäuser Verlag; Basel, Switzerland: 1995.
-
- Benowitz NL, Jacob P., III Clin Pharmacol Ther. 1994;56(5):483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

