Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells
- PMID: 20097675
- PMCID: PMC2883893
- DOI: 10.1093/cvr/cvq024
Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells
Abstract
Aims: Bone marrow-derived smooth muscle cells (BM-SMCs) have high potential as an autologous cell source of vascular progenitors but normal cell function and turnover frequency may decline with age. In this study we set out to study the effects of organismal ageing on the molecular and functional properties of BM-SMCs.
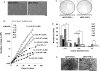
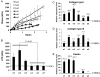
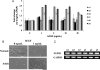
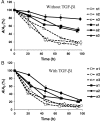
Methods and results: To address this issue, we employed a smooth muscle alpha-actin promoter (alphaSMA) driving expression of enhanced green fluorescence protein (EGFP) to isolate SMCs from bone marrow of neonatal (nBM-SMCs) or adult (aBM-SMCs) sheep and examined their proliferation potential and contractility. Compared with nBM-SMCs, aBM-SMCs exhibited lower clonogenicity and proliferation potential that could be improved significantly by addition of basic fibroblast growth factor. Vascular constructs from aBM-SMCs showed reduced ability to generate force and contract fibrin hydrogels and this function could be partially restored by addition of transforming growth factor-beta1. They also exhibited lower receptor- and non-receptor-mediated vascular contractility and mechanical strength, which was comparable to that of tissue constructs prepared with vascular SMCs from neonatal umbilical veins. In agreement with the contractile properties and mechanical strength of vascular constructs, aBM-SMCs displayed significantly lower expression of alphaSMA, smoothelin, desmin, type I collagen, and tropoelastin transcripts compared with nBM-SMCs.
Conclusion: Understanding the effects of organismal ageing on BM-SMCs and the properties of the resulting vascular constructs may lead to innovative ways to facilitate application of these cells in the treatment of cardiovascular disease which is especially prevalent in the elderly.
Figures


Similar articles
-
Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates.Cardiovasc Res. 2012 Dec 1;96(3):391-400. doi: 10.1093/cvr/cvs253. Epub 2012 Aug 31. Cardiovasc Res. 2012. PMID: 22941255 Free PMC article.
-
Phenotype-based selection of bone marrow mesenchymal stem cell-derived smooth muscle cells for elastic matrix regenerative repair in abdominal aortic aneurysms.J Tissue Eng Regen Med. 2018 Jan;12(1):e60-e70. doi: 10.1002/term.2349. Epub 2017 Apr 25. J Tissue Eng Regen Med. 2018. PMID: 27860330
-
Contractile smooth muscle cells derived from hair-follicle stem cells.Cardiovasc Res. 2008 Jul 1;79(1):24-33. doi: 10.1093/cvr/cvn059. Epub 2008 Mar 3. Cardiovasc Res. 2008. PMID: 18316325
-
Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain.Mol Cell Biomech. 2006 Mar;3(1):21-34. Mol Cell Biomech. 2006. PMID: 16711069 Review.
-
Circulating smooth muscle progenitor cells in arterial remodeling.J Mol Cell Cardiol. 2011 Feb;50(2):273-9. doi: 10.1016/j.yjmcc.2010.10.030. Epub 2010 Nov 1. J Mol Cell Cardiol. 2011. PMID: 21047514 Review.
Cited by
-
Restoring extracellular matrix synthesis in senescent stem cells.FASEB J. 2019 Oct;33(10):10954-10965. doi: 10.1096/fj.201900377R. Epub 2019 Jul 9. FASEB J. 2019. PMID: 31287964 Free PMC article.
-
NANOG Restores Contractility of Mesenchymal Stem Cell-Based Senescent Microtissues.Tissue Eng Part A. 2017 Jun;23(11-12):535-545. doi: 10.1089/ten.TEA.2016.0494. Epub 2017 Feb 28. Tissue Eng Part A. 2017. PMID: 28125933 Free PMC article.
-
Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation.PLoS One. 2011;6(8):e23232. doi: 10.1371/journal.pone.0023232. Epub 2011 Aug 30. PLoS One. 2011. PMID: 21912590 Free PMC article.
-
Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates.Cardiovasc Res. 2012 Dec 1;96(3):391-400. doi: 10.1093/cvr/cvs253. Epub 2012 Aug 31. Cardiovasc Res. 2012. PMID: 22941255 Free PMC article.
-
Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential.Stem Cells. 2012 Dec;30(12):2746-59. doi: 10.1002/stem.1223. Stem Cells. 2012. PMID: 22949105 Free PMC article.
References
-
- Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. - PubMed
-
- Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. - PubMed
-
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. - PubMed
-
- Hacia JG, Lee CC, Jimenez DF, Karaman MW, Ho VV, Siegmund KD, et al. Age-related gene expression profiles of rhesus monkey bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2008;103:1198–1210. - PubMed
-
- Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

