Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression
- PMID: 20097678
- PMCID: PMC2846162
- DOI: 10.1093/hmg/ddq026
Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression
Abstract
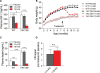
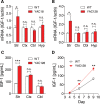
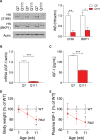
Levels of full-length huntingtin (FL htt) influence organ and body weight, independent of polyglutamine length. The growth hormone-insulin like growth factor-1 (GH-IGF-1) axis is well established as a regulator of organ growth and body weight. In this study, we investigate the involvement of the IGF-1 pathway in mediating the effect of htt on body weight. IGF-1 expression was examined in transgenic mouse lines expressing different levels of FL wild-type (WT) htt (YAC18 mice), FL mutant htt (YAC128 and BACHD mice) and truncated mutant htt (shortstop mice). We demonstrate that htt influences body weight by modulating the IGF-1 pathway. Plasma IGF-1 levels correlate with body weight and htt levels in the transgenic YAC mice expressing human htt. The effect of htt on IGF-1 expression is independent of CAG size. No effect on body weight is observed in transgenic YAC mice expressing a truncated N-terminal htt fragment (shortstop), indicating that FL htt is required for the modulation of IGF-1 expression. Treatment with 17beta-estradiol (17beta-ED) lowers the levels of circulating IGF-1 in mammals. Treatment of YAC128 with 17beta-ED, but not placebo, reduces plasma IGF-1 levels and decreases the body weight of YAC128 animals to WT levels. Furthermore, given the ubiquitous expression of IGF-1 within the central nervous system, we also examined the impact of FL htt levels on IGF-1 expression in different regions of the brain, including the striatum, cerebellum of YAC18, YAC128 and littermate WT mice. We demonstrate that the levels of FL htt influence IGF-1 expression in striatal tissues. Our data identify a novel function for FL htt in influencing IGF-1 expression.
Figures

Similar articles
-
Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington's disease.Neurobiol Dis. 2008 Jul;31(1):80-8. doi: 10.1016/j.nbd.2008.03.010. Epub 2008 Apr 16. Neurobiol Dis. 2008. PMID: 18502655 Free PMC article.
-
Wild-type huntingtin ameliorates striatal neuronal atrophy but does not prevent other abnormalities in the YAC128 mouse model of Huntington disease.BMC Neurosci. 2006 Dec 5;7:80. doi: 10.1186/1471-2202-7-80. BMC Neurosci. 2006. PMID: 17147801 Free PMC article.
-
Selective degeneration and nuclear localization of mutant huntingtin in the YAC128 mouse model of Huntington disease.Hum Mol Genet. 2005 Dec 15;14(24):3823-35. doi: 10.1093/hmg/ddi407. Epub 2005 Nov 8. Hum Mol Genet. 2005. PMID: 16278236
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
-
Polyglutamine-modulated striatal calpain activity in YAC transgenic huntington disease mouse model: impact on NMDA receptor function and toxicity.J Neurosci. 2008 Nov 26;28(48):12725-35. doi: 10.1523/JNEUROSCI.4619-08.2008. J Neurosci. 2008. PMID: 19036965 Free PMC article.
Cited by
-
Genetic manipulations of mutant huntingtin in mice: new insights into Huntington's disease pathogenesis.FEBS J. 2013 Sep;280(18):4382-94. doi: 10.1111/febs.12418. Epub 2013 Jul 31. FEBS J. 2013. PMID: 23829302 Free PMC article. Review.
-
The Huntington disease protein accelerates breast tumour development and metastasis through ErbB2/HER2 signalling.EMBO Mol Med. 2013 Feb;5(2):309-25. doi: 10.1002/emmm.201201546. Epub 2013 Jan 9. EMBO Mol Med. 2013. PMID: 23300147 Free PMC article.
-
Cognitive dysfunction in Huntington's disease: mechanisms and therapeutic strategies beyond BDNF.Brain Pathol. 2016 Nov;26(6):752-771. doi: 10.1111/bpa.12432. Brain Pathol. 2016. PMID: 27529673 Free PMC article. Review.
-
IGF-1 intranasal administration rescues Huntington's disease phenotypes in YAC128 mice.Mol Neurobiol. 2014 Jun;49(3):1126-42. doi: 10.1007/s12035-013-8585-5. Epub 2013 Dec 18. Mol Neurobiol. 2014. PMID: 24347322
-
Effects of deletion of mutant huntingtin in steroidogenic factor 1 neurons on the psychiatric and metabolic phenotype in the BACHD mouse model of Huntington disease.PLoS One. 2014 Oct 1;9(10):e107691. doi: 10.1371/journal.pone.0107691. eCollection 2014. PLoS One. 2014. PMID: 25271967 Free PMC article.
References
-
- Harper P.S. Huntington's Disease. London: W.B. Saunders; 1996.
-
- van der Burg J.M., Björkqvist M., Brundin P. Beyond the brain: widespread pathology in Huntington's disease. Lancet Neurol. 2009;8:765–774. - PubMed
-
- Cattaneo E., Zuccato C., Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat. Rev. Neurosci. 2005;6:919–930. - PubMed
-
- Rubinsztein D.C. Lessons from animal models of Huntington's disease. Trends Genet. 2002;18:202–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

