{beta}-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants
- PMID: 20097708
- PMCID: PMC2841536
- DOI: 10.1210/jc.2009-2289
{beta}-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants
Abstract
Context: beta-Cell secretory capacity, a measure of functional beta-cell mass, is often impaired in islet transplant recipients, likely because of a low engrafted beta-cell mass, although calcineurin inhibitor toxicity is often cited as the explanation.
Objective: We sought to determine whether use of the calcineurin inhibitor tacrolimus was associated with reduced beta-cell secretory capacity or with increased beta-cell secretory demand independent of engrafted islet mass.
Design and participants: We compared metabolic measures in five intraportal islet recipients vs. 10 normal controls and in seven portally-drained pancreas-kidney and eight nondiabetic kidney recipients vs. nine kidney donor controls. All transplant groups received comparable exposure to tacrolimus, and each transplant group was matched for kidney function to its respective control group.
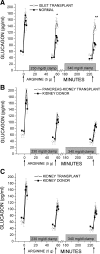
Intervention and main outcome measures: All participants underwent glucose-potentiated arginine testing where acute insulin responses to arginine (5 g) were determined under fasting (AIR(arg)), 230 mg/dl (AIR(pot)), and 340 mg/dl (AIR(max)) clamp conditions, and AIR(max) gives the beta-cell secretory capacity. Insulin sensitivity (M/I) and proinsulin secretory ratios (PISRs) were assessed to determine whether tacrolimus increased beta-cell secretory demand.
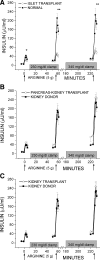
Results: Insulin responses were significantly lower than normal in the islet group for AIR(arg) (P < 0.05), AIR(pot) (P < 0.01), and AIR(max) (P < 0.01), whereas responses in the pancreas-kidney and kidney transplant groups were not different than in the kidney donor group. M/I and PISRs were not different in any of the transplant vs. control groups.
Conclusions: Impaired beta-cell secretory capacity in islet transplantation is best explained by a low engrafted beta-cell mass and not by a deleterious effect of tacrolimus.
Figures
Similar articles
-
Effect of glucagon-like peptide-1 on beta- and alpha-cell function in isolated islet and whole pancreas transplant recipients.J Clin Endocrinol Metab. 2009 Jan;94(1):181-9. doi: 10.1210/jc.2008-1806. Epub 2008 Oct 28. J Clin Endocrinol Metab. 2009. PMID: 18957498 Free PMC article.
-
Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity.J Clin Endocrinol Metab. 2007 Jul;92(7):2410-4. doi: 10.1210/jc.2007-0172. Epub 2007 May 8. J Clin Endocrinol Metab. 2007. PMID: 17488791
-
Acute insulin responses to glucose and arginine as predictors of beta-cell secretory capacity in human islet transplantation.Transplantation. 2007 Nov 27;84(10):1357-60. doi: 10.1097/01.tp.0000287595.16442.a7. Transplantation. 2007. PMID: 18049122
-
Diminished insulin secretory reserve in diabetic pancreas transplant and nondiabetic kidney transplant recipients.Diabetes. 1994 Apr;43(4):593-8. doi: 10.2337/diab.43.4.593. Diabetes. 1994. PMID: 8138066
-
Assessment of islet function following islet and pancreas transplantation.Curr Diab Rep. 2006 Aug;6(4):316-22. doi: 10.1007/s11892-006-0067-y. Curr Diab Rep. 2006. PMID: 16879785 Free PMC article. Review.
Cited by
-
Improvement in outcomes of clinical islet transplantation: 1999-2010.Diabetes Care. 2012 Jul;35(7):1436-45. doi: 10.2337/dc12-0063. Diabetes Care. 2012. PMID: 22723582 Free PMC article. Clinical Trial.
-
Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes.Endocr Rev. 2016 Feb;37(1):37-61. doi: 10.1210/er.2015-1084. Epub 2015 Dec 9. Endocr Rev. 2016. PMID: 26650437 Free PMC article. Review.
-
High residual C-peptide likely contributes to glycemic control in type 1 diabetes.J Clin Invest. 2020 Apr 1;130(4):1850-1862. doi: 10.1172/JCI134057. J Clin Invest. 2020. PMID: 31895699 Free PMC article.
-
β-Cell Secretory Capacity Predicts Metabolic Outcomes Over 6 Years After Human Islet Transplantation.Diabetes. 2025 May 1;74(5):749-759. doi: 10.2337/db24-0729. Diabetes. 2025. PMID: 39630971
-
Early-phase insulin secretion during mixed-meal tolerance testing predicts β-cell function and secretory capacity in cystic fibrosis.Front Endocrinol (Lausanne). 2024 Feb 20;15:1340346. doi: 10.3389/fendo.2024.1340346. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38444582 Free PMC article.
References
-
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR 2006 International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330 - PubMed
-
- Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL 2005 β-Cell function following human islet transplantation for type 1 diabetes. Diabetes 54:100–106 - PubMed
-
- Keymeulen B, Gillard P, Mathieu C, Movahedi B, Maleux G, Delvaux G, Ysebaert D, Roep B, Vandemeulebroucke E, Marichal M, In 't Veld P, Bogdani M, Hendrieckx C, Gorus F, Ling Z, van Rood J, Pipeleers D 2006 Correlation between β cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA 103:17444–17449 - PMC - PubMed
-
- Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM 2008 An examination of β-cell function measures and their potential use for estimating β-cell mass. Diabetes Obes Metab 10 (Suppl 4):63–76 - PubMed
-
- Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, Felldin M, Foss A, Kyllönen L, Langstrom B, Nilsson B, Korsgren O, Lundgren T 2009 Positron emission tomography in clinical islet transplantation. Am J Transplant 9:2816–2824 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

