The proline-rich N-terminal domain of G18 exhibits a novel G protein regulatory function
- PMID: 20097748
- PMCID: PMC2838322
- DOI: 10.1074/jbc.M109.057174
The proline-rich N-terminal domain of G18 exhibits a novel G protein regulatory function
Abstract
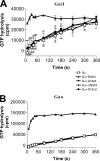
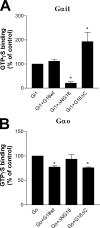
The protein G18 (also known as AGS4 or GPSM3) contains three conserved GoLoco/GPR domains in its central and C-terminal regions that bind to inactive Galpha(i), whereas the N-terminal region has not been previously characterized. We investigated whether this domain might itself regulate G protein activity by assessing the abilities of G18 and mutants thereof to modulate the nucleotide binding and hydrolytic properties of Galpha(i1) and Galpha(o). Surprisingly, in the presence of fluoroaluminate (AlF(4)(-)) both G proteins bound strongly to full-length G18 (G18wt) and to its isolated N-terminal domain (G18DeltaC) but not to its GoLoco region (DeltaNG18). Thus, it appears that its N-terminal domain promotes G18 binding to fluoroaluminate-activated Galpha(i/o). Neither G18wt nor any G18 mutant affected the GTPase activity of Galpha(i1) or Galpha(o). In contrast, complex effects were noted with respect to nucleotide binding. As inferred by the binding of [(35)S]GTPgammaS (guanosine 5'-O-[gamma-thio]triphosphate) to Galpha(i1), the isolated GoLoco region as expected acted as a guanine nucleotide dissociation inhibitor, whereas the N-terminal region exhibited a previously unknown guanine nucleotide exchange factor effect on this G protein. On the other hand, the N terminus inhibited [(35)S]GTPgammaS binding to Galpha(o), albeit to a lesser extent than the effect of the GoLoco region on Galpha(i1). Taken together, our results identify the N-terminal region of G18 as a novel G protein-interacting domain that may have distinct regulatory effects within the G(i/o) subfamily, and thus, it could potentially play a role in differentiating signals between these related G proteins.
Figures







Similar articles
-
Guanine nucleotide dissociation inhibitor activity of the triple GoLoco motif protein G18: alanine-to-aspartate mutation restores function to an inactive second GoLoco motif.Biochem J. 2004 Mar 15;378(Pt 3):801-8. doi: 10.1042/BJ20031686. Biochem J. 2004. PMID: 14656218 Free PMC article.
-
Identification and characterization of AGS4: a protein containing three G-protein regulatory motifs that regulate the activation state of Gialpha.J Biol Chem. 2004 Jun 25;279(26):27567-74. doi: 10.1074/jbc.M312786200. Epub 2004 Apr 19. J Biol Chem. 2004. PMID: 15096500
-
Inhibition of GDP/GTP exchange on G alpha subunits by proteins containing G-protein regulatory motifs.Biochemistry. 2001 May 1;40(17):5322-8. doi: 10.1021/bi015505w. Biochemistry. 2001. PMID: 11318657
-
The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits.Int J Biol Sci. 2005;1(2):51-66. doi: 10.7150/ijbs.1.51. Epub 2005 Apr 1. Int J Biol Sci. 2005. PMID: 15951850 Free PMC article. Review.
-
Return of the GDI: the GoLoco motif in cell division.Annu Rev Biochem. 2004;73:925-51. doi: 10.1146/annurev.biochem.73.011303.073756. Annu Rev Biochem. 2004. PMID: 15189163 Review.
Cited by
-
G protein signaling modulator-3: a leukocyte regulator of inflammation in health and disease.Am J Clin Exp Immunol. 2014 Aug 15;3(2):97-106. eCollection 2014. Am J Clin Exp Immunol. 2014. PMID: 25143870 Free PMC article. Review.
-
Regulation of Chemokine Signal Integration by Activator of G-Protein Signaling 4 (AGS4).J Pharmacol Exp Ther. 2017 Mar;360(3):424-433. doi: 10.1124/jpet.116.238436. Epub 2017 Jan 6. J Pharmacol Exp Ther. 2017. PMID: 28062526 Free PMC article.
-
Regulation of RGS5 GAP activity by GPSM3.Mol Cell Biochem. 2015 Jul;405(1-2):33-40. doi: 10.1007/s11010-015-2393-3. Epub 2015 Apr 5. Mol Cell Biochem. 2015. PMID: 25842189
-
Activators of G protein signaling in the kidney.J Pharmacol Exp Ther. 2015 May;353(2):235-45. doi: 10.1124/jpet.115.222695. Epub 2015 Jan 27. J Pharmacol Exp Ther. 2015. PMID: 25628392 Free PMC article. Review.
-
The Ras-binding domain region of RGS14 regulates its functional interactions with heterotrimeric G proteins.J Cell Biochem. 2013 Jun;114(6):1414-23. doi: 10.1002/jcb.24483. J Cell Biochem. 2013. PMID: 23255434 Free PMC article.
References
-
- Neves S. R., Ram P. T., Iyengar R. (2002) Science 296, 1636–1639 - PubMed
-
- Cao X., Cismowski M. J., Sato M., Blumer J. B., Lanier S. M. (2004) J. Biol. Chem. 279, 27567–27574 - PubMed
-
- Siderovski D. P., Diversé-Pierluissi M., De Vries L. (1999) Trends Biochem. Sci. 24, 340–341 - PubMed
-
- Willard F. S., Kimple R. J., Siderovski D. P. (2004) Annu. Rev. Biochem. 73, 925–951 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

