iso-DGR sequences do not mediate binding of fibronectin N-terminal modules to adherent fibronectin-null fibroblasts
- PMID: 20097751
- PMCID: PMC2838278
- DOI: 10.1074/jbc.M109.062646
iso-DGR sequences do not mediate binding of fibronectin N-terminal modules to adherent fibronectin-null fibroblasts
Abstract
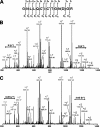
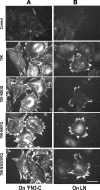
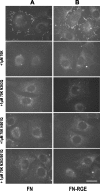
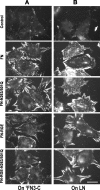
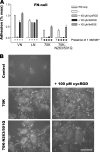
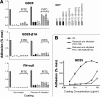
Fibronectin (FN) without an RGD sequence (FN-RGE), and thus lacking the principal binding site for alpha5beta1 integrin, is deposited into the extracellular matrix of mouse embryos. Spontaneous conversion of (263)NGR and/or (501)NGR to iso-DGR possibly explains this enigma, i.e. ligation of iso-DGR by alphavbeta3 integrin may allow cells to assemble FN. Partial modification of (263)NGR to DGR or iso-DGR was detected in purified plasma FN by mass spectrometry. To test functions of the conversion, one or both NGR sequences were mutated to QGR in recombinant N-terminal 70-kDa construct of FN (70K), full-length FN, or FN-RGE. The mutations did not affect the binding of soluble 70K to already adherent fibroblasts or the ability of soluble 70K to compete with non-mutant FN or FN-RGE for binding to FN assembly sites. Non-mutant FN and FN-N263Q/N501Q with both NGRs mutated to QGRs were assembled equally well by adherent fibroblasts. FN-RGE and FN-RGE-N263Q/N501Q were also assembled equally well. Although substrate-bound 70K mediated cell adhesion in the presence of 1 mm Mn(2+) by a mechanism that was inhibited by cyclic RGD peptide, the peptide did not inhibit 70K binding to cell surface. Mutations of the NGR sequences had no effect on Mn(2+)-enhanced cell adhesion to adsorbed 70K but caused a decrease in cell adhesion to reduced and alkylated 70K. These results demonstrate that iso-DGR sequences spontaneously converted from NGR are cryptic and do not mediate the interaction of the 70K region of FN with the cell surface during FN assembly.
Figures

Similar articles
-
IGD motifs, which are required for migration stimulatory activity of fibronectin type I modules, do not mediate binding in matrix assembly.PLoS One. 2012;7(2):e30615. doi: 10.1371/journal.pone.0030615. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355321 Free PMC article.
-
The RGD motif in fibronectin is essential for development but dispensable for fibril assembly.J Cell Biol. 2007 Jul 2;178(1):167-78. doi: 10.1083/jcb.200703021. Epub 2007 Jun 25. J Cell Biol. 2007. PMID: 17591922 Free PMC article.
-
α5β1 integrin-mediated adhesion to fibronectin is required for axis elongation and somitogenesis in mice.PLoS One. 2011;6(7):e22002. doi: 10.1371/journal.pone.0022002. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799763 Free PMC article.
-
Roles of integrins in fibronectin matrix assembly.Histol Histopathol. 1997 Jan;12(1):233-40. Histol Histopathol. 1997. PMID: 9046058 Review.
-
The ins and outs of fibronectin matrix assembly.J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
Cited by
-
Plasma and cellular fibronectin: distinct and independent functions during tissue repair.Fibrogenesis Tissue Repair. 2011 Sep 16;4:21. doi: 10.1186/1755-1536-4-21. Fibrogenesis Tissue Repair. 2011. PMID: 21923916 Free PMC article.
-
Joint inflammation related citrullination of functional arginines in extracellular proteins.Sci Rep. 2017 Aug 15;7(1):8246. doi: 10.1038/s41598-017-08597-4. Sci Rep. 2017. PMID: 28811641 Free PMC article.
-
αv-Class integrin binding to fibronectin is solely mediated by RGD and unaffected by an RGE mutation.J Cell Biol. 2020 Dec 7;219(12):e202004198. doi: 10.1083/jcb.202004198. J Cell Biol. 2020. PMID: 33141174 Free PMC article.
-
Engineering Calreticulin-Targeting Monobodies to Detect Immunogenic Cell Death in Cancer Chemotherapy.Cancers (Basel). 2021 Jun 4;13(11):2801. doi: 10.3390/cancers13112801. Cancers (Basel). 2021. PMID: 34199835 Free PMC article.
-
Extended binding site on fibronectin for the functional upstream domain of protein F1 of Streptococcus pyogenes.J Biol Chem. 2010 Dec 24;285(52):41087-99. doi: 10.1074/jbc.M110.153692. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20947497 Free PMC article.
References
-
- Pankov R., Yamada K. M. (2002) J. Cell Sci. 115, 3861–3863 - PubMed
-
- Leiss M., Beckmann K., Girós A., Costell M., Fässler R. (2008) Curr. Opin. Cell Biol. 20, 502–507 - PubMed
-
- Mao Y., Schwarzbauer J. E. (2005) Matrix Biol 24, 389–399 - PubMed
-
- Cho J., Mosher D. F. (2006) J. Thromb. Haemost 4, 1461–1469 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

