Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1)
- PMID: 20097752
- PMCID: PMC2843183
- DOI: 10.1074/jbc.M109.095505
Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1)
Abstract
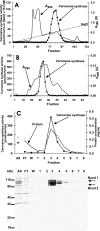
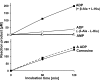
Carnosine (beta-alanyl-L-histidine) and homocarnosine (gamma-aminobutyryl-L-histidine) are abundant dipeptides in skeletal muscle and brain of most vertebrates and some invertebrates. The formation of both compounds is catalyzed by carnosine synthase, which is thought to convert ATP to AMP and inorganic pyrophosphate, and whose molecular identity is unknown. In the present work, we have purified carnosine synthase from chicken pectoral muscle about 1500-fold until only two major polypeptides of 100 and 90 kDa were present in the preparation. Mass spectrometry analysis of these polypeptides did not yield any meaningful candidate. Carnosine formation catalyzed by the purified enzyme was accompanied by a stoichiometric formation, not of AMP, but of ADP, suggesting that carnosine synthase belongs to the "ATP-grasp family" of ligases. A data base mining approach identified ATPGD1 as a likely candidate. As this protein was absent from chicken protein data bases, we reconstituted its sequence from a PCR-amplified cDNA and found it to fit with the 100-kDa polypeptide of the chicken carnosine synthase preparation. Mouse and human ATPGD1 were expressed in HEK293T cells, purified to homogeneity, and shown to catalyze the formation of carnosine, as confirmed by mass spectrometry, and of homocarnosine. Specificity studies carried out on all three enzymes were in agreement with published data. In particular, they acted with 15-25-fold higher catalytic efficiencies on beta-alanine than on gamma-aminobutyrate. The identification of the gene encoding carnosine synthase will help for a better understanding of the biological functions of carnosine and related dipeptides, which still remain largely unknown.
Figures

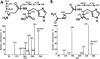
References
-
- Gulewitch W., Admiradzibi S. (1900) Ber. Dtsch. Chem. Ges. 33, 1902–1903
-
- Crush K. G. (1970) Comp. Biochem. Physiol. 34, 3–30 - PubMed
-
- Cameron J. N. (1989) J. Exp. Biol. 143, 543–548
-
- Boldyrev A. A. (2007) Carnosine and Oxidative Stress in Cells and Tissues, Nova Scientific Publisher, New York
-
- Kish S. J., Perry T. L., Hansen S. (1979) J. Neurochem. 32, 1629–1636 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

