NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia
- PMID: 20097762
- PMCID: PMC2843220
- DOI: 10.1074/jbc.M109.070706
NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia
Abstract
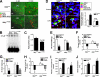
Anorexia and weight loss are prevalent in infectious diseases. To investigate the molecular mechanisms underlying these phenomena, we established animal models of infection-associated anorexia by administrating bacterial and viral products, lipopolysaccharide (LPS) and human immunodeficiency virus-1 transactivator protein (Tat). In these models, we found that the nuclear factor-kappaB (NF-kappaB), a pivotal transcription factor for inflammation-related proteins, was activated in the hypothalamus. In parallel, administration of LPS and Tat increased hypothalamic pro-inflammatory cytokine production, which was abrogated by inhibition of hypothalamic NF-kappaB. In vitro, NF-kappaB activation directly stimulated the transcriptional activity of pro-opiomelanocortin (POMC), a precursor of anorexigenic melanocortin, and mediated the stimulatory effects of LPS, Tat, and pro-inflammatory cytokines on POMC transcription, implying the involvement of NF-kappaB in controlling feeding behavior. Consistently, hypothalamic injection of LPS and Tat caused a significant reduction in food intake and body weight, which was prevented by blockade of NF-kappaB and melanocortin. Furthermore, disruption of I kappaB kinase-beta, an upstream kinase of NF-kappaB, in POMC neurons attenuated LPS- and Tat-induced anorexia. These findings suggest that infection-associated anorexia and weight loss are mediated via NF-kappaB activation in hypothalamic POMC neurons. In addition, hypothalamic NF-kappaB was activated by leptin, an important anorexigenic hormone, and mediates leptin-stimulated POMC transcription, indicating that hypothalamic NF-kappaB also serves as a downstream signaling pathway of leptin.
Figures


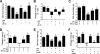

Similar articles
-
Phospholipase C-related catalytically inactive protein regulates lipopolysaccharide-induced hypothalamic inflammation-mediated anorexia in mice.Neurochem Int. 2019 Dec;131:104563. doi: 10.1016/j.neuint.2019.104563. Epub 2019 Oct 4. Neurochem Int. 2019. PMID: 31589911
-
Anorexic action of deoxynivalenol in hypothalamus and intestine.Toxicon. 2016 Aug;118:54-60. doi: 10.1016/j.toxicon.2016.04.036. Epub 2016 Apr 16. Toxicon. 2016. PMID: 27090011
-
Nuclear factor κB (NF-κB) suppresses food intake and energy expenditure in mice by directly activating the Pomc promoter.Diabetologia. 2013 Apr;56(4):925-36. doi: 10.1007/s00125-013-2831-2. Epub 2013 Jan 31. Diabetologia. 2013. PMID: 23370526
-
Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels.J Neuroendocrinol. 2018 Feb;30(2):10.1111/jne.12501. doi: 10.1111/jne.12501. J Neuroendocrinol. 2018. PMID: 28675783 Free PMC article. Review.
-
A critical update on the leptin-melanocortin system.J Neurochem. 2023 May;165(4):467-486. doi: 10.1111/jnc.15765. Epub 2023 Feb 8. J Neurochem. 2023. PMID: 36648204 Review.
Cited by
-
Lipopolysacharide Rapidly and Completely Suppresses AgRP Neuron-Mediated Food Intake in Male Mice.Endocrinology. 2016 Jun;157(6):2380-92. doi: 10.1210/en.2015-2081. Epub 2016 Apr 25. Endocrinology. 2016. PMID: 27111742 Free PMC article.
-
Hypothalamic microinflammation: a common basis of metabolic syndrome and aging.Trends Neurosci. 2015 Jan;38(1):36-44. doi: 10.1016/j.tins.2014.10.002. Epub 2014 Nov 14. Trends Neurosci. 2015. PMID: 25458920 Free PMC article. Review.
-
Increased hypothalamic serotonin turnover in inflammation-induced anorexia.BMC Neurosci. 2016 May 20;17(1):26. doi: 10.1186/s12868-016-0260-0. BMC Neurosci. 2016. PMID: 27207102 Free PMC article.
-
Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity.Cells. 2020 Jul 16;9(7):1708. doi: 10.3390/cells9071708. Cells. 2020. PMID: 32708786 Free PMC article. Review.
-
TAK1 in brain endothelial cells mediates fever and lethargy.J Exp Med. 2011 Dec 19;208(13):2615-23. doi: 10.1084/jem.20110398. Epub 2011 Dec 5. J Exp Med. 2011. PMID: 22143887 Free PMC article.
References
-
- Hart B. L. (1988) Neurosci. Biobehav. Rev. 12, 123–137 - PubMed
-
- Grunfeld C., Feingold K. R. (1992) N. Engl. J. Med. 327, 329–337 - PubMed
-
- Aubert A., Goodall G., Dantzer R. (1995) Physiol. Behav. 57, 869–873 - PubMed
-
- McCarthy D. O., Kluger M. J., Vander A. J. (1984) Am. J. Clin. Nutr. 40, 310–316 - PubMed
-
- Murray M. J., Murray A. B. (1979) Am. J. Clin. Nutr. 32, 593–596 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

