MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis
- PMID: 20097794
- PMCID: PMC2832256
- DOI: 10.1104/pp.109.147371
MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis
Abstract
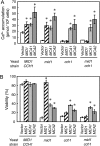
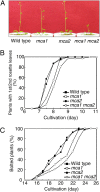
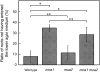
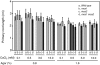
Ca(2+) is important for plant growth and development as a nutrient and a second messenger. However, the molecular nature and roles of Ca(2+)-permeable channels or transporters involved in Ca(2+) uptake in roots are largely unknown. We recently identified a candidate for the Ca(2+)-permeable mechanosensitive channel in Arabidopsis (Arabidopsis thaliana), named MCA1. Here, we investigated the only paralog of MCA1 in Arabidopsis, MCA2. cDNA of MCA2 complemented a Ca(2+) uptake deficiency in yeast cells lacking a Ca(2+) channel composed of Mid1 and Cch1. Reverse transcription polymerase chain reaction analysis indicated that MCA2 was expressed in leaves, flowers, roots, siliques, and stems, and histochemical observation showed that an MCA2 promoter::GUS fusion reporter gene was universally expressed in 10-d-old seedlings with some exceptions: it was relatively highly expressed in vascular tissues and undetectable in the cap and the elongation zone of the primary root. mca2-null plants were normal in growth and morphology. In addition, the primary root of mca2-null seedlings was able to normally sense the hardness of agar medium, unlike that of mca1-null or mca1-null mca2-null seedlings, as revealed by the two-phase agar method. Ca(2+) uptake activity was lower in the roots of mca2-null plants than those of wild-type plants. Finally, growth of mca1-null mca2-null plants was more retarded at a high concentration of Mg(2+) added to medium compared with that of mca1-null and mca2-null single mutants and wild-type plants. These results suggest that the MCA2 protein has a distinct role in Ca(2+) uptake in roots and an overlapping role with MCA1 in plant growth.
Figures




Similar articles
-
Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis.Sci Rep. 2018 Jan 11;8(1):550. doi: 10.1038/s41598-017-17483-y. Sci Rep. 2018. PMID: 29323146 Free PMC article.
-
Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3639-44. doi: 10.1073/pnas.0607703104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360695 Free PMC article.
-
Determination of structural regions important for Ca(2+) uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast.Plant Cell Physiol. 2011 Nov;52(11):1915-30. doi: 10.1093/pcp/pcr131. Epub 2011 Sep 24. Plant Cell Physiol. 2011. PMID: 21949028
-
Physiological roles of Arabidopsis MCA1 and MCA2 based on their dynamic expression patterns.J Plant Res. 2024 Sep;137(5):785-797. doi: 10.1007/s10265-024-01575-8. Epub 2024 Aug 28. J Plant Res. 2024. PMID: 39196431 Free PMC article. Review.
-
New candidates for mechano-sensitive channels potentially involved in gravity sensing in Arabidopsis thaliana.Plant Biol (Stuttg). 2014 Jan;16 Suppl 1:39-42. doi: 10.1111/plb.12044. Epub 2013 Jun 4. Plant Biol (Stuttg). 2014. PMID: 23731064 Review.
Cited by
-
Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):E5242-9. doi: 10.1073/pnas.1519555113. Epub 2016 Aug 15. Proc Natl Acad Sci U S A. 2016. PMID: 27528686 Free PMC article.
-
Cell number regulator genes in Prunus provide candidate genes for the control of fruit size in sweet and sour cherry.Mol Breed. 2013;32(2):311-326. doi: 10.1007/s11032-013-9872-6. Epub 2013 Apr 30. Mol Breed. 2013. PMID: 23976873 Free PMC article.
-
The rice "fruit-weight 2.2-like" gene family member OsFWL4 is involved in the translocation of cadmium from roots to shoots.Planta. 2018 May;247(5):1247-1260. doi: 10.1007/s00425-018-2859-0. Epub 2018 Feb 7. Planta. 2018. PMID: 29453663
-
To respond or not to respond, the recurring question in plant mechanosensitivity.Front Plant Sci. 2014 Aug 14;5:401. doi: 10.3389/fpls.2014.00401. eCollection 2014. Front Plant Sci. 2014. PMID: 25177327 Free PMC article. Review.
-
MID1-COMPLEMENTING ACTIVITY regulates cell proliferation and development via Ca2+ signaling in Marchantia polymorpha.Plant Physiol. 2024 Dec 23;197(1):kiae613. doi: 10.1093/plphys/kiae613. Plant Physiol. 2024. PMID: 39535860 Free PMC article.
References
-
- Asemaneh T, Ghaderian SM, Baker AJM. (2007) Responses to Mg/Ca balance in an Iranian serpentine endemic plant, Cleome heratensis (Capparaceae) and a related non-serpentine species, C. foliolosa. Plant Soil 293: 49–59
-
- Bechtold N, Ellis J, Pelletier G. (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci III, Sci Vie 316: 1194–1199
-
- Bechtold N, Pelletier G. (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 - PubMed
-
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA. (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119: 57–70 - PubMed
-
- Braam J. (2004) In touch: plant responses to mechanical stimuli. New Phytol 165: 373–389 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

