Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest
- PMID: 20097809
- PMCID: PMC2837996
- DOI: 10.1128/AEM.01703-09
Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest
Abstract
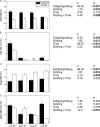
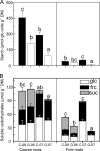
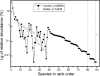
The relationships between plant carbon resources, soil carbon and nitrogen content, and ectomycorrhizal fungal (EMF) diversity in a monospecific, old-growth beech (Fagus sylvatica) forest were investigated by manipulating carbon flux by girdling. We hypothesized that disruption of the carbon supply would not affect diversity and EMF species numbers if EM fungi can be supplied by plant internal carbohydrate resources or would result in selective disappearance of EMF taxa because of differences in carbon demand of different fungi. Tree carbohydrate status, root demography, EMF colonization, and EMF taxon abundance were measured repeatedly during 1 year after girdling. Girdling did not affect root colonization but decreased EMF species richness of an estimated 79 to 90 taxa to about 40 taxa. Cenococcum geophilum, Lactarius blennius, and Tomentella lapida were dominant, colonizing about 70% of the root tips, and remained unaffected by girdling. Mainly cryptic EMF species disappeared. Therefore, the Shannon-Wiener index (H') decreased but evenness was unaffected. H' was positively correlated with glucose, fructose, and starch concentrations of fine roots and also with the ratio of dissolved organic carbon to dissolved organic nitrogen (DOC/DON), suggesting that both H' and DOC/DON were governed by changes in belowground carbon allocation. Our results suggest that beech maintains numerous rare EMF species by recent photosynthate. These EM fungi may constitute biological insurance for adaptation to changing environmental conditions. The preservation of taxa previously not known to colonize beech may, thus, form an important reservoir for future forest development.
Figures



Similar articles
-
Disproportionate abundance between ectomycorrhizal root tips and their associated mycelia.FEMS Microbiol Ecol. 2006 Nov;58(2):214-24. doi: 10.1111/j.1574-6941.2006.00166.x. FEMS Microbiol Ecol. 2006. PMID: 17064263
-
Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the eastern Himalaya.Mycorrhiza. 2010 Apr;20(4):281-7. doi: 10.1007/s00572-009-0287-5. Epub 2009 Dec 12. Mycorrhiza. 2010. PMID: 20012655 Free PMC article.
-
Ectomycorrhizal Communities on the Roots of Two Beech (Fagus sylvatica) Populations from Contrasting Climates Differ in Nitrogen Acquisition in a Common Environment.Appl Environ Microbiol. 2015 Sep 1;81(17):5957-67. doi: 10.1128/AEM.01481-15. Epub 2015 Jun 19. Appl Environ Microbiol. 2015. PMID: 26092464 Free PMC article.
-
Local Responses and Systemic Induced Resistance Mediated by Ectomycorrhizal Fungi.Front Plant Sci. 2020 Dec 14;11:590063. doi: 10.3389/fpls.2020.590063. eCollection 2020. Front Plant Sci. 2020. PMID: 33381131 Free PMC article. Review.
-
What Defines the "Kingdom" Fungi?Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.funk-0044-2017. doi: 10.1128/microbiolspec.FUNK-0044-2017. Microbiol Spectr. 2017. PMID: 28643626 Free PMC article. Review.
Cited by
-
Leaf litter species identity influences biochemical composition of ectomycorrhizal fungi.Mycorrhiza. 2019 Mar;29(2):85-96. doi: 10.1007/s00572-018-0876-2. Epub 2018 Dec 13. Mycorrhiza. 2019. PMID: 30547252
-
Interactive effects of juvenile defoliation, light conditions, and interspecific competition on growth and ectomycorrhizal colonization of Fagus sylvatica and Pinus sylvestris seedlings.Mycorrhiza. 2016 Jan;26(1):47-56. doi: 10.1007/s00572-015-0645-4. Epub 2015 May 24. Mycorrhiza. 2016. PMID: 26003665 Free PMC article.
-
Fine-Root Traits Reveal Contrasting Ecological Strategies in European Beech and Norway Spruce During Extreme Drought.Front Plant Sci. 2020 Aug 13;11:1211. doi: 10.3389/fpls.2020.01211. eCollection 2020. Front Plant Sci. 2020. PMID: 32903505 Free PMC article.
-
High Fungal Diversity but Low Seasonal Dynamics and Ectomycorrhizal Abundance in a Mountain Beech Forest.Microb Ecol. 2021 Jul;82(1):243-256. doi: 10.1007/s00248-021-01736-5. Epub 2021 Mar 23. Microb Ecol. 2021. PMID: 33755773 Free PMC article.
-
Fungal Succession During the Decomposition of Ectomycorrhizal Fine Roots.Microb Ecol. 2020 Feb;79(2):271-284. doi: 10.1007/s00248-019-01418-3. Epub 2019 Aug 8. Microb Ecol. 2020. PMID: 31392355
References
-
- Agerer, R. 1987-2006. Colour atlas of ectomycorrhizae. Einhorn-Verlag, Schwäbisch Gmünd, Germany.
-
- Agerer, R. 2001. Exploration types of ectomycorrhizae: a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107-114.
-
- Allen, A. S., J. A. Andrews, A. C. Finzi, R. Matamala, D. D. Richter, and W. H. Schlesinger. 2000. Effects of free-air CO2 enrichment (FACE) on belowground processes in a Pinus taeda forest. Ecol. Appl. 10:437-448.
-
- Allen, E. B., M. F. Allen, D. J. Helm, J. M. Trappe, R. Molina, and E. Rincon. 1995. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170:47-62.
-
- Andersen, C., I. Nikolov, P. Nikolova, R. Matyssek, and K. H. Häberle. 2005. Estimating “autotrophic” below ground respiration in spruce and beech forests: decreases following girdling. Eur. J. For. Res. 124:155-163.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

