Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts
- PMID: 20097810
- PMCID: PMC2838015
- DOI: 10.1128/AEM.02295-09
Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts
Erratum in
- Appl Environ Microbiol. 2010 Aug;76(15):5334
Abstract
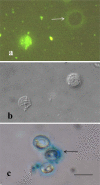
The structure and composition of the oocyst wall are primary factors determining the survival and hydrologic transport of Cryptosporidium parvum oocysts outside the host. Microscopic and biochemical analyses of whole oocysts and purified oocyst walls were undertaken to better understand the inactivation kinetics and hydrologic transport of oocysts in terrestrial and aquatic environments. Results of microscopy showed an outer electron-dense layer, a translucent middle layer, two inner electron-dense layers, and a suture structure embedded in the inner electron-dense layers. Freeze-substitution showed an expanded glycocalyx layer external to the outer bilayer, and Alcian Blue staining confirmed its presence on some but not all oocysts. Biochemical analyses of purified oocyst walls revealed carbohydrate components, medium- and long-chain fatty acids, and aliphatic hydrocarbons. Purified walls contained 7.5% total protein (by the Lowry assay), with five major bands in SDS-PAGE gels. Staining of purified oocyst walls with magnesium anilinonaphthalene-8-sulfonic acid indicated the presence of hydrophobic proteins. These structural and biochemical analyses support a model of the oocyst wall that is variably impermeable and resistant to many environmental pressures. The strength and flexibility of oocyst walls appear to depend on an inner layer of glycoprotein. The temperature-dependent permeability of oocyst walls may be associated with waxy hydrocarbons in the electron-translucent layer. The complex chemistry of these layers may explain the known acid-fast staining properties of oocysts, as well as some of the survival characteristics of oocysts in terrestrial and aquatic environments. The outer glycocalyx surface layer provides immunogenicity and attachment possibilities, and its ephemeral nature may explain the variable surface properties noted in oocyst hydrologic transport studies.
Figures



References
-
- Abrahamsen, M. S., T. J. Templeton, S. Enomoto, J. E. Abrahante, G. Zhu. C. A. Lancto, M. Deng, C. Liu, G. Widmer, S. Tzipori, G. A. Buck, P. Xu, A. T. Bankier, P. H. Dear, B. A. Konfortov, H. F. Spriggs, L. Iyer, V. Anantharaman, L. Aravind, and V. Kapur. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304:441-445. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

