Specificity of the type II secretion systems of enterotoxigenic Escherichia coli and Vibrio cholerae for heat-labile enterotoxin and cholera toxin
- PMID: 20097854
- PMCID: PMC2838057
- DOI: 10.1128/JB.01542-09
Specificity of the type II secretion systems of enterotoxigenic Escherichia coli and Vibrio cholerae for heat-labile enterotoxin and cholera toxin
Abstract
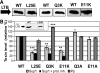
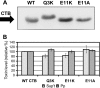
The Gram-negative type II secretion (T2S) system is a multiprotein complex mediating the release of virulence factors from a number of pathogens. While an understanding of the function of T2S components is emerging, little is known about what identifies substrates for export. To investigate T2S substrate recognition, we compared mutations affecting the secretion of two highly homologous substrates: heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli (ETEC) and cholera toxin (CT) from Vibrio cholerae. Each toxin consists of one enzymatic A subunit and a ring of five B subunits mediating the toxin's secretion. Here, we report two mutations in LT's B subunit (LTB) that reduce its secretion from ETEC without global effects on the toxin. The Q3K mutation reduced levels of secreted LT by half, and as with CT (T. D. Connell, D. J. Metzger, M. Wang, M. G. Jobling, and R. K. Holmes, Infect. Immun. 63:4091-4098, 1995), the E11K mutation impaired LT secretion. Results in vitro and in vivo show that these mutants are not degraded more readily than wild-type LT. The Q3K mutation did not significantly affect CT B subunit (CTB) secretion from V. cholerae, and the E11A mutation altered LT and CTB secretion to various extents, indicating that these toxins are identified as secretion substrates in different ways. The levels of mutant LTB expressed in V. cholerae were low or undetectable, but each CTB mutant expressed and secreted at wild-type levels in ETEC. Therefore, ETEC's T2S system seems to accommodate mutations in CTB that impair the secretion of LTB. Our results highlight the exquisitely fine-tuned relationship between T2S substrates and their coordinate secretion machineries in different bacterial species.
Figures


References
-
- Bouley, J., G. Condemine, and V. E. Shevchik. 2001. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J. Mol. Biol. 308:205-219. - PubMed
-
- Chapon, V., H. D. Simpson, X. Morelli, E. Brun, and F. Barras. 2000. Alteration of a single tryptophan residue of the cellulose-binding domain blocks secretion of the Erwinia chrysanthemi Cel5 cellulase (ex-EGZ) via the type II system. J. Mol. Biol. 303:117-123. - PubMed
-
- Chung, W. Y., R. Carter, T. Hardy, M. Sack, T. R. Hirst, and R. F. James. 2006. Inhibition of Escherichia coli heat-labile enterotoxin B subunit pentamer (EtxB5) assembly in vitro using monoclonal antibodies. J. Biol. Chem. 281:39465-39470. - PubMed
-
- Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581-588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

