SufU is an essential iron-sulfur cluster scaffold protein in Bacillus subtilis
- PMID: 20097860
- PMCID: PMC2832514
- DOI: 10.1128/JB.01536-09
SufU is an essential iron-sulfur cluster scaffold protein in Bacillus subtilis
Abstract
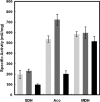
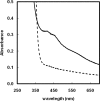
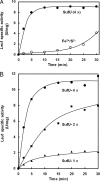
Bacteria use three distinct systems for iron-sulfur (Fe/S) cluster biogenesis: the ISC, SUF, and NIF machineries. The ISC and SUF systems are widely distributed, and many bacteria possess both of them. In Escherichia coli, ISC is the major and constitutive system, whereas SUF is induced under iron starvation and/or oxidative stress. Genomic analysis of the Fe/S cluster biosynthesis genes in Bacillus subtilis suggests that this bacterium's genome encodes only a SUF system consisting of a sufCDSUB gene cluster and a distant sufA gene. Mutant analysis of the putative Fe/S scaffold genes sufU and sufA revealed that sufU is essential for growth under minimal standard conditions, but not sufA. The drastic growth retardation of a conditional mutant depleted of SufU was coupled with a severe reduction of aconitase and succinate dehydrogenase activities in total-cell lysates, suggesting a crucial function of SufU in Fe/S protein biogenesis. Recombinant SufU was devoid of Fe/S clusters after aerobic purification. Upon in vitro reconstitution, SufU bound an Fe/S cluster with up to approximately 1.5 Fe and S per monomer. The assembled Fe/S cluster could be transferred from SufU to the apo form of isopropylmalate isomerase Leu1, rapidly forming catalytically active [4Fe-4S]-containing holo-enzyme. In contrast to native SufU, its D43A variant carried a Fe/S cluster after aerobic purification, indicating that the cluster is stabilized by this mutation. Further, we show that apo-SufU is an activator of the cysteine desulfurase SufS by enhancing its activity about 40-fold in vitro. SufS-dependent formation of holo-SufU suggests that SufU functions as an Fe/S cluster scaffold protein tightly cooperating with the SufS cysteine desulfurase.
Figures



References
-
- Agar, J. N., C. Krebs, J. Frazzon, B. H. Huynh, D. R. Dean, and M. K. Johnson. 2000. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry 39:7856-7862. - PubMed
-
- Angelini, S., C. Gerez, S. Ollagnier-de Choudens, Y. Sanakis, M. Fontecave, F. Barras, and B. Py. 2008. NfuA, a new factor required for maturing Fe/S proteins in Escherichia coli under oxidative stress and iron starvation conditions. J. Biol. Chem. 283:14084-14091. - PubMed
-
- Antonkine, M. L., E. M. Maes, R. S. Czernuszewicz, C. Breitenstein, E. Bill, C. J. Falzone, R. Balasubramanian, C. Lubner, D. A. Bryant, and J. H. Golbeck. 2007. Chemical rescue of a site-modified ligand to a [4Fe-4S] cluster in PsaC, a bacterial-like dicluster ferredoxin bound to photosystem I. Biochim. Biophys Acta 1767:712-724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

