Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules
- PMID: 20097861
- PMCID: PMC2838031
- DOI: 10.1128/JB.01231-09
Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules
Abstract
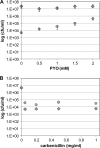
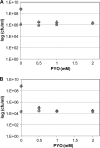
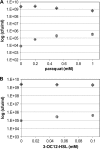
Bacterial persister cells constitute a small portion of a culture which is tolerant to killing by lethal doses of bactericidal antibiotics. These phenotypic variants are formed in numerous bacterial species, including those with clinical relevance like the opportunistic pathogen Pseudomonas aeruginosa. Although persisters are believed to contribute to difficulties in the treatment of many infectious diseases, the underlying mechanisms affecting persister formation are not well understood. Here we show that even though P. aeruginosa cultures have a significantly smaller fraction of multidrug-tolerant persister cells than cultures of Escherichia coli or Staphylococcus aureus, they can increase persister numbers in response to quorum-sensing-related signaling molecules. The phenazine pyocyanin (and the closely related molecule paraquat) and the acyl-homoserine lactone 3-OC12-HSL significantly increased the persister numbers in logarithmic P. aeruginosa PAO1 or PA14 cultures but not in E. coli or S. aureus cultures.
Figures




References
-
- Baron, S. S., G. Terranova, and J. J. Rowe. 1989. Molecular mechanism of the antimicrobial action of pyocyanin. Curr. Microbiol. 18:223-230.
-
- Bigger, J. W. 1944. Treatment of staphylococcal infections with penicillin. Lancet ii:497-500.
-
- Cao, L., R. Srikumar, and K. Poole. 2004. MexAB-OprM hyperexpression in NalC-type multidrug-resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol. Microbiol. 53:1423-1436. - PubMed
-
- Chalkley, L. J., and H. J. Koornhof. 1985. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob. Agents Chemother. 28:331-342. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

