Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition
- PMID: 20097873
- PMCID: PMC2874471
- DOI: 10.1096/fj.09-151639
Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition
Abstract
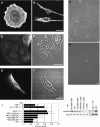
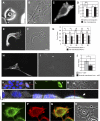
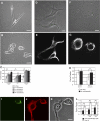
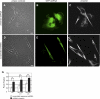
Vimentin is used widely as a marker of the epithelial to mesenchymal transitions (EMTs) that take place during embryogenesis and metastasis, yet the functional implications of the expression of this type III intermediate filament (IF) protein are poorly understood. Using form factor analysis and quantitative Western blotting of normal, metastatic, and vimentin-null cell lines, we show that the level of expression of vimentin IFs (VIFs) correlates with mesenchymal cell shape and motile behavior. The reorganization of VIFs caused by expressing a dominant-negative mutant or by silencing vimentin with shRNA (neither of which alter microtubule or microfilament assembly) causes mesenchymal cells to adopt epithelial shapes. Following the microinjection of vimentin or transfection with vimentin cDNA, epithelial cells rapidly adopt mesenchymal shapes coincident with VIF assembly. These shape transitions are accompanied by a loss of desmosomal contacts, an increase in cell motility, and a significant increase in focal adhesion dynamics. Our results demonstrate that VIFs play a predominant role in the changes in shape, adhesion, and motility that occur during the EMT.
Figures

References
-
- Hay E D. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

