Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis
- PMID: 20097882
- PMCID: PMC2845901
- DOI: 10.1182/blood-2009-07-234757
Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis
Abstract
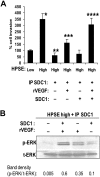
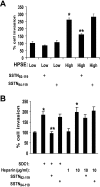
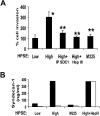
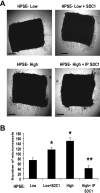
Heparanase enhances shedding of syndecan-1 (CD138), and high levels of heparanase and shed syndecan-1 in the tumor microenvironment are associated with elevated angiogenesis and poor prognosis in myeloma and other cancers. To explore how the heparanase/syndecan-1 axis regulates angiogenesis, we used myeloma cells expressing either high or low levels of heparanase and examined their impact on endothelial cell invasion and angiogenesis. Medium conditioned by heparanase-high cells significantly stimulated endothelial invasion in vitro compared with medium from heparanase-low cells. The stimulatory activity was traced to elevated levels of vascular endothelial growth factor (VEGF) and syndecan-1 in the medium. We discovered that the heparan sulfate chains of syndecan-1 captured VEGF and also attached the syndecan-1/VEGF complex to the extracellular matrix where it then stimulated endothelial invasion. In addition to its heparan sulfate chains, the core protein of syndecan-1 was also required because endothelial invasion was blocked by addition of synstatin, a peptide mimic of the integrin activating region present on the syndecan-1 core protein. These results reveal a novel mechanistic pathway driven by heparanase expression in myeloma cells whereby elevated levels of VEGF and shed syndecan-1 form matrix-anchored complexes that together activate integrin and VEGF receptors on adjacent endothelial cells thereby stimulating tumor angiogenesis.
Figures
Similar articles
-
SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis.Clin Cancer Res. 2011 Mar 15;17(6):1382-93. doi: 10.1158/1078-0432.CCR-10-2476. Epub 2011 Jan 21. Clin Cancer Res. 2011. PMID: 21257720 Free PMC article.
-
Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway.Biochem Biophys Res Commun. 2017 Apr 1;485(2):432-439. doi: 10.1016/j.bbrc.2017.02.060. Epub 2017 Feb 13. Biochem Biophys Res Commun. 2017. PMID: 28209511
-
Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis.J Biol Chem. 2007 May 4;282(18):13326-33. doi: 10.1074/jbc.M611259200. Epub 2007 Mar 8. J Biol Chem. 2007. PMID: 17347152
-
Heparanase-enhanced Shedding of Syndecan-1 and Its Role in Driving Disease Pathogenesis and Progression.J Histochem Cytochem. 2020 Dec;68(12):823-840. doi: 10.1369/0022155420937087. Epub 2020 Jul 6. J Histochem Cytochem. 2020. PMID: 32623935 Free PMC article. Review.
-
Heparanase: busy at the cell surface.Trends Biochem Sci. 2009 Oct;34(10):511-9. doi: 10.1016/j.tibs.2009.06.005. Epub 2009 Sep 3. Trends Biochem Sci. 2009. PMID: 19733083 Free PMC article. Review.
Cited by
-
Mechanisms of heparanase inhibitors in cancer therapy.Exp Hematol. 2016 Nov;44(11):1002-1012. doi: 10.1016/j.exphem.2016.08.006. Epub 2016 Aug 26. Exp Hematol. 2016. PMID: 27576132 Free PMC article. Review.
-
Influence of Fibroblasts on Mammary Gland Development, Breast Cancer Microenvironment Remodeling, and Cancer Cell Dissemination.Cancers (Basel). 2020 Jun 26;12(6):1697. doi: 10.3390/cancers12061697. Cancers (Basel). 2020. PMID: 32604738 Free PMC article. Review.
-
Differential Impact of Membrane-Bound and Soluble Forms of the Prognostic Marker Syndecan-1 on the Invasiveness, Migration, Apoptosis, and Proliferation of Cervical Cancer Cells.Front Oncol. 2022 Jan 27;12:803899. doi: 10.3389/fonc.2022.803899. eCollection 2022. Front Oncol. 2022. PMID: 35155241 Free PMC article.
-
S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis.Mol Vis. 2014 Sep 10;20:1209-24. eCollection 2014. Mol Vis. 2014. PMID: 25253987 Free PMC article.
-
Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype.Matrix Biol. 2016 Sep;55:22-34. doi: 10.1016/j.matbio.2016.03.006. Epub 2016 Mar 22. Matrix Biol. 2016. PMID: 27016342 Free PMC article.
References
-
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96(5):897–905. - PubMed
-
- Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Bartlett AH, Hayashida K, Park PW. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells. 2007;24(2):153–166. - PubMed
-
- Yang Y, Yaccoby S, Liu W, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100(2):610–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

