Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia
- PMID: 20097930
- PMCID: PMC3035516
- DOI: 10.1194/jlr.M005108
Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia
Abstract
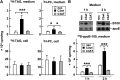
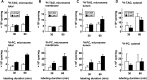
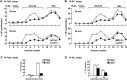
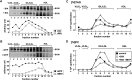
We have shown that expression of apolipoprotein (apo) C-III promotes VLDL secretion from transfected McA-RH7777 cells under lipid-rich conditions. To determine structural elements within apoC-III that confer to this function, we contrasted wild-type apoC-III with a mutant Ala23Thr originally identified in hypotriglyceridemia subjects. Although synthesis of [(3)H]glycerol-labeled TAG was comparable between cells expressing wild-type apoC-III (C3wt cells) or Ala23Thr mutant (C3AT cells), secretion of [(3)H]TAG from C3AT cells was markedly decreased. The lowered [(3)H]TAG secretion was associated with an inability of C3AT cells to assemble VLDL(1). Moreover, [(3)H]TAG within the microsomal lumen in C3AT cells was 60% higher than that in C3wt cells, yet the activity of microsomal triglyceride-transfer protein in C3AT cells was not elevated. The accumulated [(3)H]TAG in C3AT microsomal lumen was mainly associated with lumenal IDL/LDL-like lipoproteins. Phenotypically, this [(3)H]TAG fractionation profiling resembled what was observed in cells treated with brefeldin A, which at low dose specifically blocked the second-step VLDL(1) maturation. Furthermore, lumenal [(35)S]Ala23Thr protein accumulated in IDL/LDL fractions and was absent in VLDL fractions in C3AT cells. These results suggest that the presence of Ala23Thr protein in lumenal IDL/LDL particles might prevent effective fusion between lipid droplets and VLDL precursors. Thus, the current study reveals an important structural element residing within the N-terminal region of apoC-III that governs the second step VLDL(1) maturation.
Figures



Similar articles
-
Missense mutation in APOC3 within the C-terminal lipid binding domain of human ApoC-III results in impaired assembly and secretion of triacylglycerol-rich very low density lipoproteins: evidence that ApoC-III plays a major role in the formation of lipid precursors within the microsomal lumen.J Biol Chem. 2011 Aug 5;286(31):27769-80. doi: 10.1074/jbc.M110.203679. Epub 2011 Jun 15. J Biol Chem. 2011. PMID: 21676879 Free PMC article.
-
Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions.J Lipid Res. 2010 Jan;51(1):150-61. doi: 10.1194/M900346-JLR200. J Lipid Res. 2010. PMID: 19622837 Free PMC article.
-
Human apolipoprotein C-III - a new intrahepatic protein factor promoting assembly and secretion of very low density lipoproteins.Cardiovasc Hematol Disord Drug Targets. 2012 Dec;12(2):133-40. doi: 10.2174/1871529x11202020133. Cardiovasc Hematol Disord Drug Targets. 2012. PMID: 23030451 Review.
-
The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins.J Biol Chem. 1999 Sep 24;274(39):27793-800. doi: 10.1074/jbc.274.39.27793. J Biol Chem. 1999. PMID: 10488124
-
Microsome-associated lumenal lipid droplets in the regulation of lipoprotein secretion.Curr Opin Lipidol. 2013 Apr;24(2):160-70. doi: 10.1097/MOL.0b013e32835aebe7. Curr Opin Lipidol. 2013. PMID: 23123764 Review.
Cited by
-
The regulation of ApoB metabolism by insulin.Trends Endocrinol Metab. 2013 Aug;24(8):391-7. doi: 10.1016/j.tem.2013.04.001. Epub 2013 May 27. Trends Endocrinol Metab. 2013. PMID: 23721961 Free PMC article. Review.
-
Loss-of-function mutations in APOC3, triglycerides, and coronary disease.N Engl J Med. 2014 Jul 3;371(1):22-31. doi: 10.1056/NEJMoa1307095. Epub 2014 Jun 18. N Engl J Med. 2014. PMID: 24941081 Free PMC article.
-
FoxO6-mediated ApoC3 upregulation promotes hepatic steatosis and hyperlipidemia in aged rats fed a high-fat diet.Aging (Albany NY). 2024 Mar 3;16(5):4095-4115. doi: 10.18632/aging.205610. Epub 2024 Mar 3. Aging (Albany NY). 2024. PMID: 38441531 Free PMC article.
-
A Novel Role for RARα Agonists as Apolipoprotein CIII Inhibitors Identified from High Throughput Screening.Sci Rep. 2017 Jul 19;7(1):5824. doi: 10.1038/s41598-017-05163-w. Sci Rep. 2017. PMID: 28724938 Free PMC article.
-
Partner in fat metabolism: role of KLFs in fat burning and reproductive behavior.3 Biotech. 2011 Sep;1(2):59-72. doi: 10.1007/s13205-011-0016-6. Epub 2011 Jul 16. 3 Biotech. 2011. PMID: 22582147 Free PMC article.
References
-
- Jong M. C., Hofker M. H., Havekes L. M. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. - PubMed
-
- Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., Cohn J. S. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. - PubMed
-
- Schonfeld G., George P. K., Miller J., Reilly P., Witztum J. 1979. Apolipoprotein C–II and C–III levels in hyperlipoproteinemia. Metabolism. 28: 1001–1010. - PubMed
-
- Aalto-Setala K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

