Phenobarbital augments hypothermic neuroprotection
- PMID: 20098339
- PMCID: PMC2906127
- DOI: 10.1203/PDR.0b013e3181d4ff4d
Phenobarbital augments hypothermic neuroprotection
Abstract
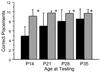
Seizures are associated with adverse outcome in infants with hypoxic-ischemic encephalopathy. We hypothesized that early administration of the anticonvulsant phenobarbital after cerebral hypoxia ischemia could enhance the neuroprotective efficacy of delayed-onset hypothermia. We tested this hypothesis in a neonatal rodent model. Seven-d-old rats (n = 104) underwent right carotid ligation, followed by 90 min 8% O2 exposure; 15 min later, they received injections of phenobarbital (40 mg/kg) or saline. One or 3 h later, all were treated with hypothermia (30 degrees C, 3 h). Function and neuropathology were evaluated after 7 d (early outcomes) or 1 mo (late outcomes). Early outcome assessment demonstrated better sensorimotor performance and less cortical damage in phenobarbital-treated groups; there were no differences between groups in which the hypothermia delay was shortened from 3 to 1 h. Late outcome assessment confirmed sustained benefits of phenobarbital + hypothermia treatment; sensorimotor performance was better (persistent attenuation of contralateral forepaw placing deficits and absence of contralateral forepaw neglect); neuropathology scores were lower (median, phenobarbital 2 and saline 8.5, p < 0.05); and less ipsilateral cerebral hemisphere %Damage (mean +/- SD, 11 +/- 17 versus 28 +/- 22, p < 0.05). These results suggest that early posthypoxia-ischemia administration of phenobarbital may augment the neuroprotective efficacy of therapeutic hypothermia.
Figures


References
-
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. - PubMed
-
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. - PubMed
-
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. - PubMed
-
- Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–1465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

