Opposing regulation of human alveolar type II cell differentiation by nitric oxide and hyperoxia
- PMID: 20098340
- PMCID: PMC3066065
- DOI: 10.1203/PDR.0b013e3181d4f20f
Opposing regulation of human alveolar type II cell differentiation by nitric oxide and hyperoxia
Abstract
Clinical trials demonstrated decreasing rates of bronchopulmonary dysplasia in preterm infants with hypoxic respiratory failure treated with inhaled nitric oxide (iNO). However, the molecular and biochemical effects of iNO on developing human fetal lungs remain vastly unknown. By using a well-characterized model of human fetal alveolar type II cells, we assessed the effects of iNO and hyperoxia, independently and concurrently, on NO-cGMP signaling pathway and differentiation. Exposure to iNO increased cGMP levels by 40-fold after 3 d and by 8-fold after 5 d despite constant expression of phosphodiesterase-5 (PDE5). The levels of cGMP declined significantly on exposure to iNO and hyperoxia at 3 and 5 d, although expression of soluble guanylyl cyclase (sGC) was sustained. Surfactant proteins B and C (SP-B, SP-C) and thyroid transcription factor (TTF)-1 mRNA levels increased in cells exposed to iNO in normoxia but not on exposure to iNO plus hyperoxia. Collectively, these data indicate an increase in type II cell markers when undifferentiated lung epithelial cells are exposed to iNO in room air. However, hyperoxia overrides these potentially beneficial effects of iNO despite sustained expression of sGC.
Figures
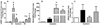
 ), or hyperoxia plus NO (
), or hyperoxia plus NO ( ); *p < 0.05 compared to normoxia on day 3 and †p < 0.05 compared to corresponding treatment group on day 3, n=4 for all treatment groups.
); *p < 0.05 compared to normoxia on day 3 and †p < 0.05 compared to corresponding treatment group on day 3, n=4 for all treatment groups.
 ), or hyperoxia plus NO (
), or hyperoxia plus NO ( ).
).
 ), or hyperoxia plus NO (
), or hyperoxia plus NO ( ).
).
 ), or hyperoxia plus NO (
), or hyperoxia plus NO ( ); *p < 0.05, n=3 for all treatment groups.
); *p < 0.05, n=3 for all treatment groups.
 ), or hyperoxia plus NO (
), or hyperoxia plus NO ( ); data is expressed as percent of the corresponding normoxia control *p < 0.05, n=5 for all treatment groups.
); data is expressed as percent of the corresponding normoxia control *p < 0.05, n=5 for all treatment groups.References
-
- Ballard PL. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989;10:165–181. - PubMed
-
- Beers MF, Shuman H, Liley HG, Floros J, Gonzales LW, Yue N, Ballard PL. Surfactant protein B in human fetal lung: developmental and glucocorticoid regulation. Pediatr Res. 1995;38:668–675. - PubMed
-
- Guttentag SH, Beers MF, Bieler BM, Ballard PL. Surfactant protein B processing in human fetal lung. Am J Physiol. 1998;275:L559–L566. - PubMed
-
- Solarin KO, Ballard PL, Guttentag SH, Lomax CA, Beers MF. Expression and glucocorticoid regulation of surfactant protein C in human fetal lung. Pediatr Res. 1997;42:356–364. - PubMed
-
- Stevenson DK, Wright LL, Lemons JA, Oh W, Korones SB, Papile LA, Bauer CR, Stoll BJ, Tyson JE, Shankaran S, Fanaroff AA, Donovan EF, Ehrenkranz RA, Verter J. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632–1639. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

