Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza
- PMID: 20098442
- PMCID: PMC2822886
- DOI: 10.1038/ni.1838
Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza
Erratum in
- Nat Immunol. 2010 Jul;11(7):644
Abstract
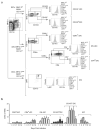
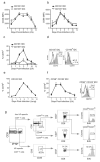
The question of which dendritic cells (DCs) respond to pulmonary antigens and cross-prime CD8(+) T cells remains controversial. We show here that influenza-specific CD8(+) T cell priming was controlled by different DCs at different times after infection. Whereas early priming was controlled by both CD103(+)CD11b(lo) and CD103(-)CD11b(hi) DCs, CD103(-)CD11b(hi) DCs dominated antigen presentation at the peak of infection. Moreover, CD103(-)CD11b(hi) DCs captured exogenous antigens in the lungs and directly cross-primed CD8(+) T cells in the draining lymph nodes without transferring antigen to CD8alpha(+) DCs. Finally, we show that CD103(-)CD11b(hi) DCs were the only DCs to express CD70 after influenza infection and that CD70 expression on CD103(-)CD11b(hi) DCs licensed them to expand CD8(+) T cell populations responding to both influenza and exogenous ovalbumin.
Figures






References
-
- Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. - PubMed
-
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
-
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. - PubMed
-
- Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

