The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis: a pilot study
- PMID: 20098520
- PMCID: PMC2805564
- DOI: 10.4088/PCC.08m00749bro
The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis: a pilot study
Abstract
Objective: To evaluate the efficacy and safety of eszopiclone 3 mg, a nonbenzodiazepine medication/hypnotic indicated for the treatment of insomnia with comorbid rheumatoid arthritis (RA).
Method: This multicenter, double-blind, placebo-controlled pilot study was conducted in 153 patients aged 25-64 years with American College of Rheumatology-defined RA who met DSM-IV criteria for insomnia. The data were collected from February to November of 2004. Patients were randomly assigned to either eszopiclone or placebo nightly for 4 weeks, followed by a 2-week placebo run out. Efficacy was evaluated using patient reports of sleep (wake time after sleep onset [WASO], sleep latency [SL], and total sleep time [TST]), daytime function, pain, and RA assessments. Insomnia severity was evaluated using the Insomnia Severity Index. Safety was also evaluated.
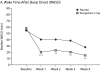
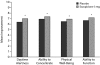
Results: Eszopiclone significantly improved all patient-reported sleep measures (WASO, SL, and TST), sleep quality, depth of sleep, and daytime function (P < .05 vs placebo). At week 4, 48% of eszopiclone-treated patients had no clinically meaningful insomnia as assessed by ISI score (versus 30% of placebo-treated patients, P = .03). Eszopiclone was significantly better than placebo on some RA-associated pain measures: (1) overall (P = .05), pain (P = .006), and pain and other symptoms (P = .02) scores of the Arthritis Self-Efficacy Scale, (2) tender joint counts (P = .03) and pain severity scores (P = .023), (3) the activities domain of the Health Assessment Questionnaire-Disability Index (P = .04), and (4) the role physical (P = .03) and bodily pain (P = .01) scales of the 36-item Medical Outcomes Study Short-Form General Health Survey. The most commonly reported adverse events with eszopiclone were unpleasant taste and transient increases in RA symptoms.
Conclusions: In this pilot study of patients with insomnia comorbid with RA, eszopiclone 3 mg improved all assessed sleep and daytime function measures over the treatment period, as well as some measures of RA-associated pain, disability, and quality of life.
Trial registration: clinicaltrials.gov Identifier: NCT00367965.
Figures







References
-
- Centers for Disease Control and Prevention. Health-related quality of life among adults with arthritis: Behavioral Risk Factor Surveillance System, 11 states, 1996–1998. MMWR Morb Mortal Wkly Rep. 2000;49(17):366–369. - PubMed
-
- Hagen KB, Kvien TK, Bjorndal A. Musculoskeletal pain and quality of life in patients with noninflammatory joint pain compared to rheumatoid arthritis: a population survey. J Rheumatol. 1997;24(9):1703–1709. - PubMed
-
- Hill CL, Parsons J, Taylor A, et al. Health related quality of life in a population sample with arthritis. J Rheumatol. 1999;26(9):2029–2035. - PubMed
-
- Devins GM, Edworthy SM, Paul LC, et al. Restless sleep, illness intrusiveness, and depressive symptoms in three chronic illness conditions: rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. J Psychosom Res. 1993;37(2):163–170. - PubMed
-
- Drewes AM, Nielsen KD, Hansen B, et al. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology (Oxford) 2000;39(11):1287–1289. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
