The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa
- PMID: 20098607
- PMCID: PMC2810220
- DOI: 10.3390/md7040689
The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa
Abstract
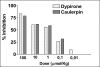
The antinociceptive and anti-inflammatory activity of caulerpin was investigated. This bisindole alkaloid was isolated from the lipoid extract of Caulerpa racemosa and its structure was identified by spectroscopic methods, including IR and NMR techniques. The pharmacological assays used were the writhing and the hot plate tests, the formalin-induced pain, the capsaicin-induced ear edema and the carrageenan-induced peritonitis. Caulerpin was given orally at a concentration of 100 micromol/kg. In the abdominal constriction test caulerpin showed reduction in the acetic acid-induced nociception at 0.0945 micromol (0.0103-1.0984) and for dypirone it was 0.0426 micromol (0.0092-0.1972). In the hot plate test in vivo the inhibition of nociception by caulerpin (100 micromol/kg, p.o.) was also favorable. This result suggests that this compound exhibits a central activity, without changing the motor activity (seen in the rotarod test). Caulerpin (100 micromol/kg, p.o.) reduced the formalin effects in both phases by 35.4% and 45.6%, respectively. The possible anti-inflammatory activity observed in the second phase in the formalin test of caulerpin (100 micromol/kg, p.o.) was confirmed on the capsaicin-induced ear edema model, where an inhibition of 55.8% was presented. Indeed, it was also observed in the carrageenan-induced peritonitis that caulerpin (100 micromol/kg, p.o.) exhibited anti-inflammatory activity, reducing significantly the number of recruit cells by 48.3%. Pharmacological studies are continuing in order to characterize the mechanism(s) responsible for the antinociceptive and anti-inflammatory actions and also to identify other active principles present in Caulerpa racemosa.
Keywords: Caulerpa racemosa; anti-inflammatory; antinociceptive; caulerpin.
Figures




References
-
- Barbosa-Filho JM, Cunha EVL, Gray AI. Alkaloids of the Menispermaceae. In: Cordell GA, editor. The Alkaloids. Vol. 54. Academic Press; Illinois, USA: 2000. pp. 1–199.
-
- Barbosa-Filho JM, Sette IMF, Cunha EVL, Guedes DN, Silva MS. Protoberberine alkaloids. In: Cordell GA, editor. The Alkaloids. Vol. 62. Elsevier Inc; San Diego, CA, USA: 2005. pp. 1–75. - PubMed
-
- Conserva LM, Pereira CAB, Barbosa-Filho JM. Alkaloids of the Hernandiaceae: Occurrence and a Compilation of their Biological Activities. In: Cordell GA, editor. The Alkaloids. Vol. 62. Elsevier; San Diego, CA, USA: 2005. pp. 175–243. - PubMed
-
- Cunha EVL, Barbosa-Filho JM. Alcalóides derivados do núcleo isoquinolínico. In: Yunes RA, Cechinel-Filho V, editors. Química de Produtos Naturais, Novos Fármacos e a Moderna Farmacognosia. 2a ed. Univali Editora; Itajaí-PR, Brazil: 2009. pp. 279–319.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
