A submarine journey: the pyrrole-imidazole alkaloids
- PMID: 20098608
- PMCID: PMC2810223
- DOI: 10.3390/md7040705
A submarine journey: the pyrrole-imidazole alkaloids
Abstract
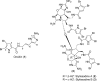
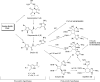
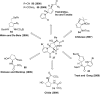
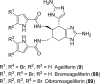
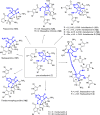
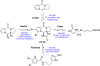
In his most celebrated tale "The Picture of Dorian Gray", Oscar Wilde stated that "those who go beneath the surface do so at their peril". This sentence could be a prophetical warning for the practitioner who voluntarily challenges himself with trying to synthesize marine sponge-deriving pyrrole-imidazole alkaloids. This now nearly triple-digit membered community has been growing exponentially in the last 20 years, both in terms of new representatives and topological complexity--from simple, achiral oroidin to the breathtaking 12-ring stylissadines A and B, each possessing 16 stereocenters. While the biosynthesis and the role in the sponge economy of most of these alkaloids still lies in the realm of speculations, significant biological activities for some of them have clearly emerged. This review will account for the progress in achieving the total synthesis of the more biologically enticing members of this class of natural products.
Keywords: marine sponges; oroidin; pyrrole-imidazole alkaloids; total synthesis.
Figures

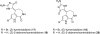
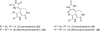
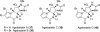
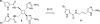
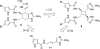
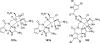
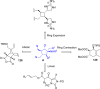


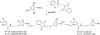


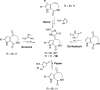
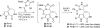
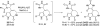
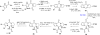
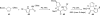

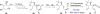
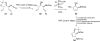
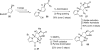
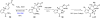
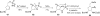
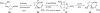
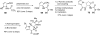
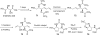

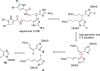

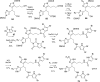


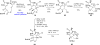
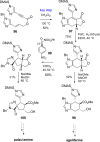
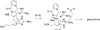
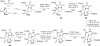


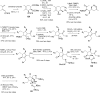
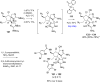
References
-
- Wan Y, Hur W, Cho CY, Liu Y, Adrian FJ, Lozach O, Bach S, Mayer T, Fabbro D, Meijer L, Gray NS. Synthesis and target identification of hymenialdisine analogs. Chem Biol. 2004;11:247–259. - PubMed
-
- Hoffmann H, Lindel T. Synthesis of the pyrrole-imidazole alkaloids. Synthesis. 2003:1753–1783.
-
- Jacquot DEN, Lindel T. Challenge palau'amine: Current standings. Curr Org Chem. 2005;9:1551–1565.
-
- Jin Z. Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat Prod Rep. 2005;22:196–229. - PubMed
-
- Jin Z. Imidazole, oxazole and thiazole alkaloids. Nat Prod Rep. 2006;23:464–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
