Biochemistry of Meiotic Recombination: Formation, Processing, and Resolution of Recombination Intermediates
- PMID: 20098639
- PMCID: PMC2809983
- DOI: 10.1007/7050_2008_039
Biochemistry of Meiotic Recombination: Formation, Processing, and Resolution of Recombination Intermediates
Abstract
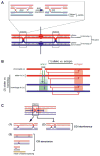
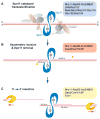
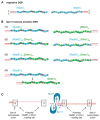
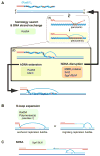
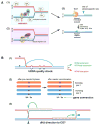
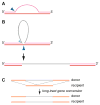
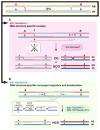
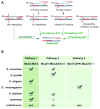
Meiotic recombination ensures accurate chromosome segregation during the first meiotic division and provides a mechanism to increase genetic heterogeneity among the meiotic products. Unlike homologous recombination in somatic (vegetative) cells, where sister chromatid interactions prevail and crossover formation is avoided, meiotic recombination is targeted to involve homologs, resulting in crossovers to connect the homologs before anaphase of the first meiotic division. The mechanisms responsible for homolog choice and crossover control are poorly understood, but likely involve meiosis-specific recombination proteins, as well as meiosis-specific chromosome organization and architecture. Much progress has been made to identify and biochemically characterize many of the proteins acting during meiotic recombination. This review will focus on the proteins that generate and process heteroduplex DNA, as well as those that process DNA junctions during meiotic recombination, with particular attention to how recombination activities promote crossover resolution between homologs.
Figures




Similar articles
-
Inhibition of the Smc5/6 complex during meiosis perturbs joint molecule formation and resolution without significantly changing crossover or non-crossover levels.PLoS Genet. 2013 Nov;9(11):e1003898. doi: 10.1371/journal.pgen.1003898. Epub 2013 Nov 7. PLoS Genet. 2013. PMID: 24244180 Free PMC article.
-
Rad51-mediated interhomolog recombination during budding yeast meiosis is promoted by the meiotic recombination checkpoint and the conserved Pif1 helicase.PLoS Genet. 2022 Dec 12;18(12):e1010407. doi: 10.1371/journal.pgen.1010407. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36508468 Free PMC article.
-
The Cdc14 Phosphatase Controls Resolution of Recombination Intermediates and Crossover Formation during Meiosis.Int J Mol Sci. 2021 Sep 10;22(18):9811. doi: 10.3390/ijms22189811. Int J Mol Sci. 2021. PMID: 34575966 Free PMC article.
-
Meiosis.WormBook. 2017 May 4;2017:1-43. doi: 10.1895/wormbook.1.178.1. WormBook. 2017. PMID: 26694509 Free PMC article. Review.
-
Crossover or non-crossover outcomes: tailored processing of homologous recombination intermediates.Curr Opin Genet Dev. 2021 Dec;71:39-47. doi: 10.1016/j.gde.2021.06.012. Epub 2021 Jul 20. Curr Opin Genet Dev. 2021. PMID: 34293660 Review.
Cited by
-
The Control of the Crossover Localization in Allium.Int J Mol Sci. 2023 Apr 11;24(8):7066. doi: 10.3390/ijms24087066. Int J Mol Sci. 2023. PMID: 37108228 Free PMC article.
-
Saccharomyces cerevisiae Dmc1 and Rad51 proteins preferentially function with Tid1 and Rad54 proteins, respectively, to promote DNA strand invasion during genetic recombination.J Biol Chem. 2012 Aug 17;287(34):28727-37. doi: 10.1074/jbc.M112.373290. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22761450 Free PMC article.
-
Noncanonical Contributions of MutLγ to VDE-Initiated Crossovers During Saccharomyces cerevisiae Meiosis.G3 (Bethesda). 2019 May 7;9(5):1647-1654. doi: 10.1534/g3.119.400150. G3 (Bethesda). 2019. PMID: 30902890 Free PMC article.
-
Msh4 and Msh5 function in SC-independent chiasma formation during the streamlined meiosis of Tetrahymena.Genetics. 2014 Nov;198(3):983-93. doi: 10.1534/genetics.114.169698. Epub 2014 Sep 11. Genetics. 2014. PMID: 25217051 Free PMC article.
-
Unresolved Recombination Intermediates Cause a RAD9-Dependent Cell Cycle Arrest in Saccharomyces cerevisiae.Genetics. 2019 Nov;213(3):805-818. doi: 10.1534/genetics.119.302632. Epub 2019 Sep 27. Genetics. 2019. PMID: 31562181 Free PMC article.
References
-
- Adams MD, McVey M, Sekelsky JJ. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265–267. - PubMed
-
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggest an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
