The efficiency of contact lens care regimens on protein removal from hydrogel and silicone hydrogel lenses
- PMID: 20098668
- PMCID: PMC2808856
The efficiency of contact lens care regimens on protein removal from hydrogel and silicone hydrogel lenses
Abstract
Purpose: To investigate the efficiency of lysozyme and albumin removal from silicone hydrogel and conventional contact lenses, using a polyhexamethylene biguanide multipurpose solution (MPS) in a soaking or rubbing/soaking application and a hydrogen peroxide system (H(2)O(2)).
Methods: Etafilcon A, lotrafilcon B and balafilcon A materials were incubated in protein solutions for up to 14 days. Lenses were either placed in radiolabeled protein to quantify the amount deposited or in fluorescent-conjugated protein to identify its location, using confocal laser scanning microscopy (CLSM). Lenses were either rinsed with PBS or soaked overnight in H(2)O(2) or MPS with and without lens rubbing.
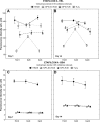
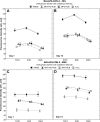
Results: After 14 days lysozyme was highest on etafilcon A (2,200 mug) >balafilcon A (50 microg) >lotrafilcon B (9.7 microg) and albumin was highest on balafilcon A (1.9 microg) =lotrafilcon B (1.8 microg) >etafilcon A (0.2 microg). Lysozyme removal was greatest for balafilcon A >etafilcon A >lotrafilcon B, with etafilcon A showing the most change in protein distribution. Albumin removal was highest from etafilcon A >balafilcon A >lotrafilcon B. H(2)O(2) exhibited greater lysozyme removal from etafilcon A compared to both MPS procedures (p<0.001) but performed similarly for lotrafilcon B and balafilcon A lenses (p>0.62). Albumin removal was solely material specific, while all care regimens performed to a similar degree (p>0.69).
Conclusions: Protein removal efficiency for the regimens evaluated depended on the lens material and protein type. Overall, lens rubbing with MPS before soaking did not reduce the protein content on the lenses compared to nonrubbed lenses (p=0.89).
Figures

Similar articles
-
Composition of incubation solution impacts in vitro protein uptake to silicone hydrogel contact lenses.Mol Vis. 2012;18:337-47. Epub 2012 Feb 4. Mol Vis. 2012. PMID: 22355245 Free PMC article.
-
Kinetics of lysozyme activity recovered from conventional and silicone hydrogel contact lens materials.J Biomater Sci Polym Ed. 2010;21(3):343-58. doi: 10.1163/156856209X415873. J Biomater Sci Polym Ed. 2010. PMID: 20178690
-
Localization of lysozyme sorption to conventional and silicone hydrogel contact lenses using confocal microscopy.Curr Eye Res. 2009 Aug;34(8):683-97. doi: 10.1080/02713680903015900. Curr Eye Res. 2009. PMID: 19899996
-
Uptake and release of polyhexamethylene biguanide (PHMB) from hydrogel and silicone hydrogel contact lenses using a radiolabel methodology.Cont Lens Anterior Eye. 2022 Oct;45(5):101575. doi: 10.1016/j.clae.2022.101575. Epub 2022 Feb 4. Cont Lens Anterior Eye. 2022. PMID: 35131120
-
Multipurpose Lens Care Systems and Silicone Hydrogel Contact Lens Wettability: A Systematic Review.Eye Contact Lens. 2022 Sep 1;48(9):356-361. doi: 10.1097/ICL.0000000000000914. Epub 2022 Jun 30. Eye Contact Lens. 2022. PMID: 36002943
Cited by
-
Biological and Clinical Implications of Lysozyme Deposition on Soft Contact Lenses.Optom Vis Sci. 2015 Jul;92(7):750-7. doi: 10.1097/OPX.0000000000000615. Optom Vis Sci. 2015. PMID: 26002002 Free PMC article. Review.
-
Composition of incubation solution impacts in vitro protein uptake to silicone hydrogel contact lenses.Mol Vis. 2012;18:337-47. Epub 2012 Feb 4. Mol Vis. 2012. PMID: 22355245 Free PMC article.
-
The effect of fluorescent labels on protein sorption in polymer hydrogels.J Fluoresc. 2014 Nov;24(6):1639-50. doi: 10.1007/s10895-014-1450-8. Epub 2014 Sep 12. J Fluoresc. 2014. PMID: 25209202
-
In vitro Evaluation of the Location of Cholesteryl Ester Deposits on Monthly Replacement Silicone Hydrogel Contact Lens Materials.Clin Ophthalmol. 2020 Sep 24;14:2821-2828. doi: 10.2147/OPTH.S270575. eCollection 2020. Clin Ophthalmol. 2020. PMID: 33061266 Free PMC article.
-
Activity of Deposited Lysozyme on Contemporary Soft Contact Lenses Exposed to Differing Lens Care Systems.Clin Ophthalmol. 2021 Apr 23;15:1727-1733. doi: 10.2147/OPTH.S296116. eCollection 2021. Clin Ophthalmol. 2021. PMID: 33935489 Free PMC article.
References
-
- Neff AN, Caldwell KD. Property modification. In: Von Recum A, Jacobi JE, editors. Handbook of biomaterials evaluation: Scientific, technical, and clinical testing of implant materials. 2nd ed. Philadelphia, PA: Taylor & Francis; 1999. p. 201–26.
-
- Holmberg M, Hou X. Competitive protein adsorption - multilayer adsorption and surface induced protein aggregation. Langmuir. 2009;25:2081–9. - PubMed
-
- Brennan NA, Coles ML. Deposits and symptomatology with soft contact lens wear. Int Contact Lens Clin. 2000;27:75–100.
-
- Jones L, Evans K, Sariri R, Franklin V, Tighe B. Lipid and protein deposition of N-vinyl pyrrolidone-containing group II and group IV frequent replacement contact lenses. CLAO J. 1997;23:122–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
