Molecular interaction studies of trimethoxy flavone with human serum albumin
- PMID: 20098677
- PMCID: PMC2809094
- DOI: 10.1371/journal.pone.0008834
Molecular interaction studies of trimethoxy flavone with human serum albumin
Abstract
Background: Human serum albumin (HSA) is the most abundant protein in blood plasma, having high affinity binding sites for several endogenous and exogenous compounds. Trimethoxy flavone (TMF) is a naturally occurring flavone isolated from Andrographis viscosula and used in the treatment of dyspepsia, influenza, malaria, respiratory functions and as an astringent and antidote for poisonous stings of some insects.
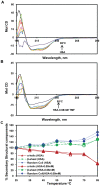
Methodology/principal findings: The main aim of the experiment was to examine the interaction between TMF and HSA at physiological conditions. Upon addition of TMF to HSA, the fluorescence emission was quenched and the binding constant of TMF with HSA was found to be K(TMF) = 1.0+/-0.01x10(3) M(-1), which corresponds to -5.4 kcal M(-1) of free energy. Micro-TOF Q mass spectrometry results showed a mass increase of from 66,513 Da (free HSA) to 66,823 Da (HAS +Drug), indicating the strong binding of TMF with HSA resulting in decrease of fluorescence. The HSA conformation was altered upon binding of TMF to HSA with decrease in alpha-helix and an increase in beta-sheets and random coils suggesting partial unfolding of protein secondary structure. Molecular docking experiments found that TMF binds strongly with HSA at IIIA domain of hydrophobic pocket with hydrogen bond and hydrophobic interactions. Among which two hydrogen bonds are formed between O (19) of TMF to Arg 410, Tyr 411 and another one from O (7) of TMF to Asn 391, with bond distance of 2.1 A, 3.6 A and 2.6 A, respectively.
Conclusions/significance: In view of the evidence presented, it is imperative to assign a greater role of HSA's as a carrier molecule for many drugs to understand the interactions of HSA with TMF will be pivotal in the design of new TMF-inspired drugs.
Conflict of interest statement
Figures




References
-
- Yang CS, Landau JM, Huang HMT. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. - PubMed
-
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. - PubMed
-
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. - PubMed
-
- Amic D, Davidovic-Amic D, Beslo D, Trinajstic N. Structure-radical scavenging activity relationships of flavonoids. Croatica chemica acta. 2003;76:55–61.
-
- Husain SR, Cillard J, Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry. 1987;26:2489–2491.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

