Two multiplex assays that simultaneously identify 22 possible mutation sites in the KRAS, BRAF, NRAS and PIK3CA genes
- PMID: 20098682
- PMCID: PMC2809099
- DOI: 10.1371/journal.pone.0008802
Two multiplex assays that simultaneously identify 22 possible mutation sites in the KRAS, BRAF, NRAS and PIK3CA genes
Abstract
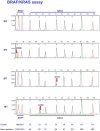
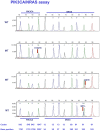
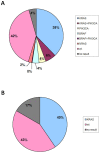
Recently a number of randomized trials have shown that patients with advanced colorectal cancer do not benefit from therapies targeting the epidermal growth factor receptor when their tumors harbor mutations in the KRAS, BRAF and PIK3CA genes. We developed two multiplex assays that simultaneously screen 22 nucleotides in the KRAS, NRAS, BRAF and PIK3CA genes for mutations. The assays were validated on 294 tumor DNA samples from patients with advanced colorectal cancer. In these samples 119 KRAS codon 12 and 13 mutations had been identified by sequence analysis, 126 tumors were wild-type for KRAS and the analysis failed in 49 of the 294 samples due to poor DNA quality. The two mutation assays detected 130 KRAS mutations, among which were 3 codon 61 mutations, and in addition 32 PIK3CA, 13 BRAF and 6 NRAS mutations. In 19 tumors a KRAS mutation was found together with a mutation in the PIK3CA gene. One tumor was mutant for both PIK3CA and BRAF. In summary, the mutations assays identified 161 tumors with a mutation, 120 were wild-type and the analysis failed in 13. The material cost of the 2 mutation assays was calculated to be 8-fold lower than the cost of sequencing required to obtain the same data. In addition, the mutation assays are less labor intensive. We conclude that the performance of the two multiplex mutation assays was superior to direct sequencing. In addition, these assays are cheaper and easier to interpret. The assays may also be of use for selection of patients with other tumor types.
Conflict of interest statement
Figures
References
-
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. - PubMed
-
- Raponi M, Winkler H, Dracopoli NC. KRAS mutations predict response to EGFR inhibitors. Curr Opin Pharmacol. 2008;8:413–418. - PubMed
-
- Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. - PubMed
-
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

