Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance
- PMID: 20098690
- PMCID: PMC2809107
- DOI: 10.1371/journal.pone.0008817
Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance
Abstract
Background: The sequence of events leading to the development of insulin resistance (IR) as well as the underlying pathophysiological mechanisms are incompletely understood. As reductionist approaches have been largely unsuccessful in providing an understanding of the pathogenesis of IR, there is a need for an integrative, time-resolved approach to elucidate the development of the disease.
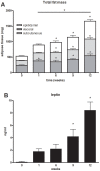
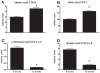
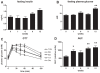
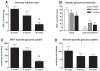
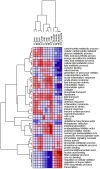
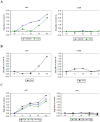
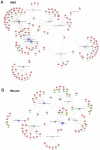
Methodology/principal findings: Male ApoE3Leiden transgenic mice exhibiting a humanized lipid metabolism were fed a high-fat diet (HFD) for 0, 1, 6, 9, or 12 weeks. Development of IR was monitored in individual mice over time by performing glucose tolerance tests and measuring specific biomarkers in plasma, and hyperinsulinemic-euglycemic clamp analysis to assess IR in a tissue-specific manner. To elucidate the dynamics and tissue-specificity of metabolic and inflammatory processes key to IR development, a time-resolved systems analysis of gene expression and metabolite levels in liver, white adipose tissue (WAT), and muscle was performed. During HFD feeding, the mice became increasingly obese and showed a gradual increase in glucose intolerance. IR became first manifest in liver (week 6) and then in WAT (week 12), while skeletal muscle remained insulin-sensitive. Microarray analysis showed rapid upregulation of carbohydrate (only liver) and lipid metabolism genes (liver, WAT). Metabolomics revealed significant changes in the ratio of saturated to polyunsaturated fatty acids (liver, WAT, plasma) and in the concentrations of glucose, gluconeogenesis and Krebs cycle metabolites, and branched amino acids (liver). HFD evoked an early hepatic inflammatory response which then gradually declined to near baseline. By contrast, inflammation in WAT increased over time, reaching highest values in week 12. In skeletal muscle, carbohydrate metabolism, lipid metabolism, and inflammation was gradually suppressed with HFD.
Conclusions/significance: HFD-induced IR is a time- and tissue-dependent process that starts in liver and proceeds in WAT. IR development is paralleled by tissue-specific gene expression changes, metabolic adjustments, changes in lipid composition, and inflammatory responses in liver and WAT involving p65-NFkB and SOCS3. The alterations in skeletal muscle are largely opposite to those in liver and WAT.
Conflict of interest statement
Figures




References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
-
- Herder C, Kolb H, Koenig W, Haastert B, Muller-Scholze S, et al. Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg, Survey 4 (KORA S4). Diabetes Care. 2006;29:368–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

