Lactococcus lactis, an alternative system for functional expression of peripheral and intrinsic Arabidopsis membrane proteins
- PMID: 20098692
- PMCID: PMC2808337
- DOI: 10.1371/journal.pone.0008746
Lactococcus lactis, an alternative system for functional expression of peripheral and intrinsic Arabidopsis membrane proteins
Abstract
Background: Despite their functional and biotechnological importance, the study of membrane proteins remains difficult due to their hydrophobicity and their low natural abundance in cells. Furthermore, into established heterologous systems, these proteins are frequently only produced at very low levels, toxic and mis- or unfolded. Lactococcus lactis, a gram-positive lactic bacterium, has been traditionally used in food fermentations. This expression system is also widely used in biotechnology for large-scale production of heterologous proteins. Various expression vectors, based either on constitutive or inducible promoters, are available for this system. While previously used to produce bacterial and eukaryotic membrane proteins, the ability of this system to produce plant membrane proteins was until now not tested.
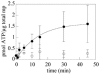
Methodology/principal findings: The aim of this work was to test the expression, in Lactococcus lactis, of either peripheral or intrinsic Arabidopsis membrane proteins that could not be produced, or in too low amount, using more classical heterologous expression systems. In an effort to easily transfer genes from Gateway-based Arabidopsis cDNA libraries to the L. lactis expression vector pNZ8148, we first established a cloning strategy compatible with Gateway entry vectors. Interestingly, the six tested Arabidopsis membrane proteins could be produced, in Lactococcus lactis, at levels compatible with further biochemical analyses. We then successfully developed solubilization and purification processes for three of these proteins. Finally, we questioned the functionality of a peripheral and an intrinsic membrane protein, and demonstrated that both proteins were active when produced in this system.
Conclusions/significance: Altogether, these data suggest that Lactococcus lactis might be an attractive system for the efficient and functional production of difficult plant membrane proteins.
Conflict of interest statement
Figures



Similar articles
-
Functional expression of plant membrane proteins in Lactococcus lactis.Methods Mol Biol. 2015;1258:147-65. doi: 10.1007/978-1-4939-2205-5_8. Methods Mol Biol. 2015. PMID: 25447863 Review.
-
Membrane Protein Production in Lactococcus lactis for Functional Studies.Methods Mol Biol. 2016;1432:79-101. doi: 10.1007/978-1-4939-3637-3_6. Methods Mol Biol. 2016. PMID: 27485331
-
Membrane protein expression in Lactococcus lactis.Methods Mol Biol. 2010;601:67-85. doi: 10.1007/978-1-60761-344-2_5. Methods Mol Biol. 2010. PMID: 20099140
-
Lactococcus lactis is an Efficient Expression System for Mammalian Membrane Proteins Involved in Liver Detoxification, CYP3A4, and MGST1.Mol Biotechnol. 2016 Apr;58(4):299-310. doi: 10.1007/s12033-016-9928-z. Mol Biotechnol. 2016. PMID: 26961909
-
10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis.Appl Microbiol Biotechnol. 2005 Oct;68(6):705-17. doi: 10.1007/s00253-005-0107-6. Epub 2005 Oct 13. Appl Microbiol Biotechnol. 2005. PMID: 16088349 Review.
Cited by
-
Formation of intracellular vesicles within the Gram+ Lactococcus lactis induced by the overexpression of Caveolin-1β.Microb Cell Fact. 2022 Nov 17;21(1):239. doi: 10.1186/s12934-022-01944-9. Microb Cell Fact. 2022. PMID: 36384643 Free PMC article.
-
Functional expression of an orchid fragrance gene in Lactococcus lactis.Int J Mol Sci. 2012;13(2):1582-1597. doi: 10.3390/ijms13021582. Epub 2012 Feb 2. Int J Mol Sci. 2012. PMID: 22408409 Free PMC article.
-
A Lactococcus lactis expression vector set with multiple affinity tags to facilitate isolation and direct labeling of heterologous secreted proteins.Appl Microbiol Biotechnol. 2017 Nov;101(22):8139-8149. doi: 10.1007/s00253-017-8524-x. Epub 2017 Oct 2. Appl Microbiol Biotechnol. 2017. PMID: 28971274 Free PMC article.
-
Rational design strategies for functional reconstitution of plant cytochrome P450s in microbial systems.Curr Opin Plant Biol. 2021 Apr;60:102005. doi: 10.1016/j.pbi.2021.102005. Epub 2021 Feb 26. Curr Opin Plant Biol. 2021. PMID: 33647811 Free PMC article. Review.
-
Oligomeric status and nucleotide binding properties of the plastid ATP/ADP transporter 1: toward a molecular understanding of the transport mechanism.PLoS One. 2012;7(3):e32325. doi: 10.1371/journal.pone.0032325. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438876 Free PMC article.
References
-
- Lacapere JJ, Pebay-Peyroula E, Neumann JM, Etchebest C. Determining membrane protein structures: still a challenge. Trends Biochem Sci. 2007;32:259–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

