GABA increases electrical excitability in a subset of human unmyelinated peripheral axons
- PMID: 20098693
- PMCID: PMC2808338
- DOI: 10.1371/journal.pone.0008780
GABA increases electrical excitability in a subset of human unmyelinated peripheral axons
Abstract
Background: A proportion of small diameter primary sensory neurones innervating human skin are chemosensitive. They respond in a receptor dependent manner to chemical mediators of inflammation as well as naturally occurring algogens, thermogens and pruritogens. The neurotransmitter GABA is interesting in this respect because in animal models of neuropathic pain GABA pre-synaptically regulates nociceptive input to the spinal cord. However, the effect of GABA on human peripheral unmyelinated axons has not been established.
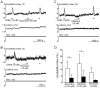
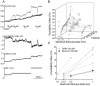
Methodology/principal findings: Electrical stimulation was used to assess the effect of GABA on the electrical excitability of unmyelinated axons in isolated fascicles of human sural nerve. GABA (0.1-100 microM) increased electrical excitability in a subset (ca. 40%) of C-fibres in human sural nerve fascicles suggesting that axonal GABA sensitivity is selectively restricted to a sub-population of human unmyelinated axons. The effects of GABA were mediated by GABA(A) receptors, being mimicked by bath application of the GABA(A) agonist muscimol (0.1-30 microM) while the GABA(B) agonist baclofen (10-30 microM) was without effect. Increases in excitability produced by GABA (10-30 microM) were blocked by the GABA(A) antagonists gabazine (10-20 microM), bicuculline (10-20 microM) and picrotoxin (10-20 microM).
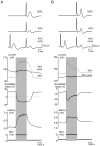
Conclusions/significance: Functional GABA(A) receptors are present on a subset of unmyelinated primary afferents in humans and their activation depolarizes these axons, an effect likely due to an elevated intra-axonal chloride concentration. GABA(A) receptor modulation may therefore regulate segmental and peripheral components of nociception.
Conflict of interest statement
Figures


References
-
- Brown DA. Extrasynaptic GABA systems. TINS. 1979;2:271–273.
-
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. - PubMed
-
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. - PubMed
-
- Valeyev AY, Hackman JC, Wood PM, Davidoff RA. Pharmacologically novel GABA receptor in human dorsal root ganglion neurons. J Neurophysiol. 1996;76:3555–3558. - PubMed
-
- Valeyev AY, Hackman JC, Holohean AM, Wood PM, Katz JL, Davidoff RA. GABA-Induced Cl− current in cultured embryonic human dorsal root ganglion neurons. J Neurophysiol. 1999;82:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

