A phase II trial of the epothilone B analog ixabepilone (BMS-247550) in patients with metastatic melanoma
- PMID: 20098694
- PMCID: PMC2808339
- DOI: 10.1371/journal.pone.0008714
A phase II trial of the epothilone B analog ixabepilone (BMS-247550) in patients with metastatic melanoma
Abstract
Background: Ixabepilone (BMS-247550), an epothilone B analog, is a microtubule stabilizing agent which has shown activity in several different tumor types and preclinical models in melanoma. In an open label, one-arm, multi-center phase II trial the efficacy and toxicity of this epothilone was investigated in two different cohorts: chemotherapy-naïve (previously untreated) and previously treated patients with metastatic melanoma.
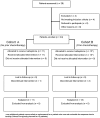
Methodology/principal findings: Eligible patients had histologically-confirmed stage IV melanoma, with an ECOG performance status of 0 to 2. Ixabepilone was administered at a dose of 20 mg/m(2) on days 1, 8, and 15 during each 28-day cycle. The primary endpoint was response rate (RR); secondary endpoints were time to progression (TTP) and toxicity. Twenty-four patients were enrolled and 23 were evaluable for response. Initial serum lactate dehydrogenase (LDH) levels were elevated in 6/11 (55%) of the previously treated and in 5/13 (38%) of the previously untreated patients. No complete or partial responses were seen in either cohort. One patient in the previously treated group developed neutropenia and fatal septic shock. Seventeen patients (8 in the previously untreated group and 9 in the previously treated group) progressed after 2 cycles, whereas six patients (3 in each group) had stable disease after 2-6 cycles. Median TTP was 1.74 months in the previously untreated group (95% CI = 1.51 months, upper limit not estimated) and 1.54 months in the previously treated group (95% CI = 1.15 months, 2.72 months). Grade 3 and/or 4 toxicities occurred in 5/11 (45%) of previously untreated and in 5/13 (38%) of previously treated patients and included neutropenia, peripheral neuropathy, fatigue, diarrhea, and dyspnea.
Conclusions/significance: Ixabepilone has no meaningful activity in either chemotherapy-naïve (previously untreated) or previously treated patients with metastatic melanoma. Further investigation with ixabepilone as single agent in the treatment of melanoma is not warranted.
Trial registration: Clinical Trials.gov NCT00036764.
Conflict of interest statement
Figures
Similar articles
-
Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes.J Clin Oncol. 2007 Aug 10;25(23):3421-7. doi: 10.1200/JCO.2006.10.0784. Epub 2007 Jul 2. J Clin Oncol. 2007. PMID: 17606971 Clinical Trial.
-
Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: a gynecologic oncology group study.J Clin Oncol. 2010 Jan 1;28(1):149-53. doi: 10.1200/JCO.2009.24.1455. Epub 2009 Nov 16. J Clin Oncol. 2010. PMID: 19917861 Free PMC article. Clinical Trial.
-
A phase II study of ixabepilone (BMS-247550) in metastatic renal-cell carcinoma.Cancer Biol Ther. 2007 Apr;6(4):490-3. doi: 10.4161/cbt.6.4.3831. Cancer Biol Ther. 2007. PMID: 17457044 Clinical Trial.
-
Ixabepilone: a novel microtubule inhibitor for the treatment of locally advanced or metastatic breast cancer.Clin Ther. 2008 Sep;30(9):1590-617. doi: 10.1016/j.clinthera.2008.09.015. Clin Ther. 2008. PMID: 18840366 Review.
-
Clinical experience with epothilones in patients with breast cancer.Clin Breast Cancer. 2008 Mar;8 Suppl 2:S71-8. doi: 10.3816/cbc.2008.s.003. Clin Breast Cancer. 2008. PMID: 18637402 Review.
Cited by
-
Small-Molecule Mitotic Inhibitors as Anticancer Agents: Discovery, Classification, Mechanisms of Action, and Clinical Trials.Int J Mol Sci. 2025 Apr 1;26(7):3279. doi: 10.3390/ijms26073279. Int J Mol Sci. 2025. PMID: 40244152 Free PMC article. Review.
-
Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents?Cancer Biol Ther. 2010 Nov 1;10(9):839-53. doi: 10.4161/cbt.10.9.13738. Epub 2010 Nov 1. Cancer Biol Ther. 2010. PMID: 20935499 Free PMC article. Review.
-
Weekly ixabepilone administration in heavily pretreated metastatic breast cancer patients.Med Oncol. 2011 Dec;28 Suppl 1:S115-20. doi: 10.1007/s12032-010-9726-6. Epub 2010 Oct 27. Med Oncol. 2011. PMID: 20978949
References
-
- Aamdal S, Wolff I, Kaplan S, Paridaens R, Kerger J, et al. Docetaxel (Taxotere) in advanced malignant melanoma: a phase II study of the EORTC Early Clinical Trials Group. Eur J Cancer. 1994;30A:1061–1064. - PubMed
-
- Bedikian AY, Plager C, Papadopoulos N, Eton O, Ellerhorst J, et al. Phase II evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res. 2004;14:63–66. - PubMed
-
- Bedikian AY, Weiss GR, Legha SS, Burris HA, 3rd, Eckardt JR, et al. Phase II trial of docetaxel in patients with advanced cutaneous malignant melanoma previously untreated with chemotherapy. J Clin Oncol. 1995;13:2895–2899. - PubMed
-
- Einzig AI, Hochster H, Wiernik PH, Trump DL, Dutcher JP, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9:59–64. - PubMed
-
- Einzig AI, Schuchter LM, Recio A, Coatsworth S, Rodriquez R, et al. Phase II trial of docetaxel (Taxotere) in patients with metastatic melanoma previously untreated with cytotoxic chemotherapy. Med Oncol. 1996;13:111–117. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical