A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution
- PMID: 20098722
- PMCID: PMC2808208
- DOI: 10.1371/journal.pbio.1000282
A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution
Abstract
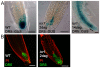
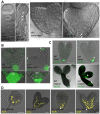
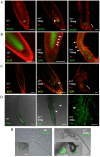
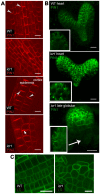
Development in multicellular organisms depends on the ability of individual cells to coordinate their behavior by means of small signaling molecules to form correctly patterned tissues. In plants, a unique mechanism of directional transport of the signaling molecule auxin between cells connects cell polarity and tissue patterning and thus is required for many aspects of plant development. Direction of auxin flow is determined by polar subcellular localization of PIN auxin efflux transporters. Dynamic PIN polar localization results from the constitutive endocytic cycling to and from the plasma membrane, but it is not well understood how this mechanism connects to regulators of cell polarity. The Rho family small GTPases ROPs/RACs are master regulators of cell polarity, however their role in regulating polar protein trafficking and polar auxin transport has not been established. Here, by analysis of mutants and transgenic plants, we show that the ROP interactor and polarity regulator scaffold protein ICR1 is required for recruitment of PIN proteins to the polar domains at the plasma membrane. icr1 mutant embryos and plants display an a array of severe developmental aberrations that are caused by compromised differential auxin distribution. ICR1 functions at the plasma membrane where it is required for exocytosis but does not recycle together with PINs. ICR1 expression is quickly induced by auxin but is suppressed at the positions of stable auxin maxima in the hypophysis and later in the embryonic and mature root meristems. Our results imply that ICR1 is part of an auxin regulated positive feedback loop realized by a unique integration of auxin-dependent transcriptional regulation into ROP-mediated modulation of cell polarity. Thus, ICR1 forms an auxin-modulated link between cell polarity, exocytosis, and auxin transport-dependent tissue patterning.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

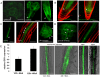
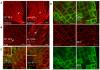

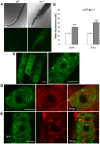
Comment in
-
An auxin regulated positive feedback loop integrates Rho modulated cell polarity with pattern formation.Plant Signal Behav. 2010 Jun;5(6):709-11. doi: 10.4161/psb.5.6.11644. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20383060 Free PMC article.
Similar articles
-
An auxin regulated positive feedback loop integrates Rho modulated cell polarity with pattern formation.Plant Signal Behav. 2010 Jun;5(6):709-11. doi: 10.4161/psb.5.6.11644. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20383060 Free PMC article.
-
Bimodal regulation of ICR1 levels generates self-organizing auxin distribution.Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):E5471-9. doi: 10.1073/pnas.1413918111. Epub 2014 Dec 2. Proc Natl Acad Sci U S A. 2014. PMID: 25468974 Free PMC article.
-
WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity.PLoS Genet. 2018 Jan 29;14(1):e1007177. doi: 10.1371/journal.pgen.1007177. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29377885 Free PMC article.
-
Subcellular trafficking of PIN auxin efflux carriers in auxin transport.Eur J Cell Biol. 2010 Feb-Mar;89(2-3):231-5. doi: 10.1016/j.ejcb.2009.11.003. Epub 2009 Nov 26. Eur J Cell Biol. 2010. PMID: 19944476 Review.
-
Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development.Cell Mol Life Sci. 2006 Dec;63(23):2738-54. doi: 10.1007/s00018-006-6116-5. Cell Mol Life Sci. 2006. PMID: 17013565 Free PMC article. Review.
Cited by
-
Microtubule-associated ROP interactors affect microtubule dynamics and modulate cell wall patterning and root hair growth.Development. 2022 Nov 15;149(22):dev200811. doi: 10.1242/dev.200811. Epub 2022 Nov 16. Development. 2022. PMID: 36314989 Free PMC article.
-
Developmentally distinct activities of the exocyst enable rapid cell elongation and determine meristem size during primary root growth in Arabidopsis.BMC Plant Biol. 2014 Dec 31;14:386. doi: 10.1186/s12870-014-0386-0. BMC Plant Biol. 2014. PMID: 25551204 Free PMC article.
-
The RAC/ROP GTPase activator OsRopGEF10 functions in crown root development by regulating cytokinin signaling in rice.Plant Cell. 2023 Jan 2;35(1):453-468. doi: 10.1093/plcell/koac297. Plant Cell. 2023. PMID: 36190337 Free PMC article.
-
Lily Cdc42/Rac-interactive binding motif-containing protein, a Rop target, involves calcium influx and phosphoproteins during pollen germination and tube growth.Plant Signal Behav. 2010 Nov;5(11):1460-3. doi: 10.4161/psb.5.11.13466. Epub 2010 Nov 1. Plant Signal Behav. 2010. PMID: 21060254 Free PMC article.
-
Isolation and characterization of OsMY1, a putative partner of OsRac5 from Oryza sativa L.Mol Biol Rep. 2014 Mar;41(3):1829-36. doi: 10.1007/s11033-014-3032-x. Epub 2014 Jan 24. Mol Biol Rep. 2014. PMID: 24464125
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

