Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease
- PMID: 20098725
- PMCID: PMC2808216
- DOI: 10.1371/journal.pbio.1000291
Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease
Abstract
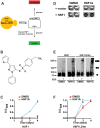
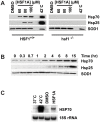
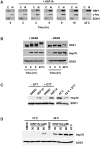
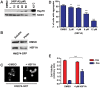
Neurodegenerative diseases such as Huntington disease are devastating disorders with no therapeutic approaches to ameliorate the underlying protein misfolding defect inherent to poly-glutamine (polyQ) proteins. Given the mounting evidence that elevated levels of protein chaperones suppress polyQ protein misfolding, the master regulator of protein chaperone gene transcription, HSF1, is an attractive target for small molecule intervention. We describe a humanized yeast-based high-throughput screen to identify small molecule activators of human HSF1. This screen is insensitive to previously characterized activators of the heat shock response that have undesirable proteotoxic activity or that inhibit Hsp90, the central chaperone for cellular signaling and proliferation. A molecule identified in this screen, HSF1A, is structurally distinct from other characterized small molecule human HSF1 activators, activates HSF1 in mammalian and fly cells, elevates protein chaperone expression, ameliorates protein misfolding and cell death in polyQ-expressing neuronal precursor cells and protects against cytotoxicity in a fly model of polyQ-mediated neurodegeneration. In addition, we show that HSF1A interacts with components of the TRiC/CCT complex, suggesting a potentially novel regulatory role for this complex in modulating HSF1 activity. These studies describe a novel approach for the identification of new classes of pharmacological interventions for protein misfolding that underlies devastating neurodegenerative disease.
Conflict of interest statement
Duke University has filed a patent application for the screening technology described in this report as well as the small molecules identified via this screening technology.
Figures


Similar articles
-
Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones.J Biol Chem. 2008 Sep 19;283(38):26188-97. doi: 10.1074/jbc.M710521200. Epub 2008 Jul 16. J Biol Chem. 2008. PMID: 18632670 Free PMC article.
-
Hsf1 on a leash - controlling the heat shock response by chaperone titration.Exp Cell Res. 2020 Nov 1;396(1):112246. doi: 10.1016/j.yexcr.2020.112246. Epub 2020 Aug 27. Exp Cell Res. 2020. PMID: 32861670 Review.
-
Identification of novel small molecule chaperone activators for neurodegenerative disease treatment.Biomed Pharmacother. 2025 Jun;187:118049. doi: 10.1016/j.biopha.2025.118049. Epub 2025 Apr 15. Biomed Pharmacother. 2025. PMID: 40239269 Free PMC article.
-
Protein phosphatase 5 is a negative modulator of heat shock factor 1.J Biol Chem. 2005 Aug 12;280(32):28989-96. doi: 10.1074/jbc.M503594200. Epub 2005 Jun 20. J Biol Chem. 2005. PMID: 15967796
-
[Molecular therapy targeting protein misfolding and aggregation for the polyglutamine diseases].Rinsho Shinkeigaku. 2009 Nov;49(11):913-6. doi: 10.5692/clinicalneurol.49.913. Rinsho Shinkeigaku. 2009. PMID: 20030247 Review. Japanese.
Cited by
-
Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson's Disease.Int J Mol Sci. 2023 Nov 12;24(22):16233. doi: 10.3390/ijms242216233. Int J Mol Sci. 2023. PMID: 38003423 Free PMC article. Review.
-
A Futile Battle? Protein Quality Control and the Stress of Aging.Dev Cell. 2018 Jan 22;44(2):139-163. doi: 10.1016/j.devcel.2017.12.020. Dev Cell. 2018. PMID: 29401418 Free PMC article. Review.
-
Optimal HSF1 activation in response to acute cold stress in BAT requires nuclear TXNIP.iScience. 2023 Apr 10;26(5):106538. doi: 10.1016/j.isci.2023.106538. eCollection 2023 May 19. iScience. 2023. PMID: 37168572 Free PMC article.
-
Chaperone co-inducer BGP-15 inhibits histone deacetylases and enhances the heat shock response through increased chromatin accessibility.Cell Stress Chaperones. 2017 Sep;22(5):717-728. doi: 10.1007/s12192-017-0798-5. Epub 2017 May 4. Cell Stress Chaperones. 2017. PMID: 28474205 Free PMC article.
-
RNA Sequence Analysis of Human Huntington Disease Brain Reveals an Extensive Increase in Inflammatory and Developmental Gene Expression.PLoS One. 2015 Dec 4;10(12):e0143563. doi: 10.1371/journal.pone.0143563. eCollection 2015. PLoS One. 2015. PMID: 26636579 Free PMC article.
References
-
- Chiti F, Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Orr H. T, Zoghbi H. Y. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. - PubMed
-
- Caughey B, Lansbury P. T. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. - PubMed
-
- Young J. C, Agashe V. R, Siegers K, Hartl F. U. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. - PubMed
-
- Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

