Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice
- PMID: 20098733
- PMCID: PMC2808242
- DOI: 10.1371/journal.pone.0008762
Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice
Abstract
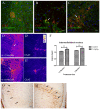
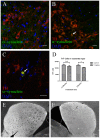
In patients with Parkinson's disease (PD), the associated pathology follows a characteristic pattern involving inter alia the enteric nervous system (ENS), the dorsal motor nucleus of the vagus (DMV), the intermediolateral nucleus of the spinal cord and the substantia nigra, providing the basis for the neuropathological staging of the disease. Here we report that intragastrically administered rotenone, a commonly used pesticide that inhibits Complex I of the mitochondrial respiratory chain, is able to reproduce PD pathological staging as found in patients. Our results show that low doses of chronically and intragastrically administered rotenone induce alpha-synuclein accumulation in all the above-mentioned nervous system structures of wild-type mice. Moreover, we also observed inflammation and alpha-synuclein phosphorylation in the ENS and DMV. HPLC analysis showed no rotenone levels in the systemic blood or the central nervous system (detection limit [rotenone]<20 nM) and mitochondrial Complex I measurements showed no systemic Complex I inhibition after 1.5 months of treatment. These alterations are sequential, appearing only in synaptically connected nervous structures, treatment time-dependent and accompanied by inflammatory signs and motor dysfunctions. These results strongly suggest that the local effect of pesticides on the ENS might be sufficient to induce PD-like progression and to reproduce the neuroanatomical and neurochemical features of PD staging. It provides new insight into how environmental factors could trigger PD and suggests a transsynaptic mechanism by which PD might spread throughout the central nervous system.
Conflict of interest statement
Figures


References
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S21–23. - PubMed
-
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486. - PubMed
-
- Crossman AR. Neural mechanisms in disorders of movement. Comp Biochem Physiol A Comp Physiol. 1989;93:141–149. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson's disease. Ann Neurol. 2004;55:761–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

