Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages
- PMID: 20098737
- PMCID: PMC2808246
- DOI: 10.1371/journal.pone.0008769
Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages
Abstract
Background: Microorganisms capable of surviving within macrophages are rare, but represent very successful pathogens. One of them is Mycobacterium tuberculosis (Mtb) whose resistance to early mechanisms of macrophage killing and failure of its phagosomes to fuse with lysosomes causes tuberculosis (TB) disease in humans. Thus, defining the mechanisms of phagosome maturation arrest and identifying mycobacterial factors responsible for it are key to rational design of novel drugs for the treatment of TB. Previous studies have shown that Mtb and the related vaccine strain, M. bovis bacille Calmette-Guérin (BCG), disrupt the normal function of host Rab5 and Rab7, two small GTPases that are instrumental in the control of phagosome fusion with early endosomes and late endosomes/lysosomes respectively.
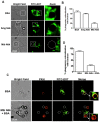
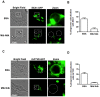
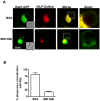
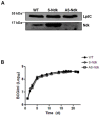
Methodology/principal findings: Here we show that recombinant Mtb nucleoside diphosphate kinase (Ndk) exhibits GTPase activating protein (GAP) activity towards Rab5 and Rab7. Then, using a model of latex bead phagosomes, we demonstrated that Ndk inhibits phagosome maturation and fusion with lysosomes in murine RAW 264.7 macrophages. Maturation arrest of phagosomes containing Ndk-beads was associated with the inactivation of both Rab5 and Rab7 as evidenced by the lack of recruitment of their respective effectors EEA1 (early endosome antigen 1) and RILP (Rab7-interacting lysosomal protein). Consistent with these findings, macrophage infection with an Ndk knocked-down BCG strain resulted in increased fusion of its phagosome with lysosomes along with decreased survival of the mutant.
Conclusion: Our findings provide evidence in support of the hypothesis that mycobacterial Ndk is a putative virulence factor that inhibits phagosome maturation and promotes survival of mycobacteria within the macrophage.
Conflict of interest statement
Figures


Similar articles
-
Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity.PLoS Pathog. 2013;9(7):e1003499. doi: 10.1371/journal.ppat.1003499. Epub 2013 Jul 18. PLoS Pathog. 2013. PMID: 23874203 Free PMC article.
-
Mycobacterium bovis BCG disrupts the interaction of Rab7 with RILP contributing to inhibition of phagosome maturation.J Leukoc Biol. 2007 Dec;82(6):1437-45. doi: 10.1189/jlb.0507289. J Leukoc Biol. 2007. PMID: 18040083
-
Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase.Mol Cell Biol. 2003 Apr;23(7):2501-14. doi: 10.1128/MCB.23.7.2501-2514.2003. Mol Cell Biol. 2003. PMID: 12640132 Free PMC article.
-
Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking.Electrophoresis. 1997 Dec;18(14):2542-7. doi: 10.1002/elps.1150181409. Electrophoresis. 1997. PMID: 9527483 Review.
-
Mycobacterial manipulation of the host cell.FEMS Microbiol Rev. 2005 Nov;29(5):1041-50. doi: 10.1016/j.femsre.2005.04.013. Epub 2005 Jul 1. FEMS Microbiol Rev. 2005. PMID: 16040149 Review.
Cited by
-
Macrophage: A Cell With Many Faces and Functions in Tuberculosis.Front Immunol. 2022 May 6;13:747799. doi: 10.3389/fimmu.2022.747799. eCollection 2022. Front Immunol. 2022. PMID: 35603185 Free PMC article. Review.
-
KefB inhibits phagosomal acidification but its role is unrelated to M. tuberculosis survival in host.Sci Rep. 2013 Dec 18;3:3527. doi: 10.1038/srep03527. Sci Rep. 2013. PMID: 24346161 Free PMC article.
-
Host-directed therapy for bacterial infections -Modulation of the phagolysosome pathway.Front Immunol. 2023 Sep 29;14:1227467. doi: 10.3389/fimmu.2023.1227467. eCollection 2023. Front Immunol. 2023. PMID: 37841276 Free PMC article. Review.
-
Mycobacterium tuberculosis and its clever approaches to escape the deadly macrophage.World J Microbiol Biotechnol. 2023 Sep 5;39(11):300. doi: 10.1007/s11274-023-03735-9. World J Microbiol Biotechnol. 2023. PMID: 37667129 Review.
-
Towards new TB vaccines.Semin Immunopathol. 2020 Jun;42(3):315-331. doi: 10.1007/s00281-020-00794-0. Epub 2020 Mar 18. Semin Immunopathol. 2020. PMID: 32189035 Free PMC article. Review.
References
-
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. - PubMed
-
- LoBue P. Extensively drug-resistant tuberculosis. Curr Opin Infect Dis. 2009;22(2):167–173. - PubMed
-
- Jozefowski S, Sobota A, Kwiatkowska K. How mycobacterium tuberculosis subverts host immune responses. Bioessays. 2008;30(10):943–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

