Brainstem circuitry regulating phasic activation of trigeminal motoneurons during REM sleep
- PMID: 20098748
- PMCID: PMC2808333
- DOI: 10.1371/journal.pone.0008788
Brainstem circuitry regulating phasic activation of trigeminal motoneurons during REM sleep
Abstract
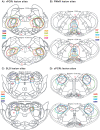
Background: Rapid eye movement sleep (REMS) is characterized by activation of the cortical and hippocampal electroencephalogram (EEG) and atonia of non-respiratory muscles with superimposed phasic activity or twitching, particularly of cranial muscles such as those of the eye, tongue, face and jaw. While phasic activity is a characteristic feature of REMS, the neural substrates driving this activity remain unresolved. Here we investigated the neural circuits underlying masseter (jaw) phasic activity during REMS. The trigeminal motor nucleus (Mo5), which controls masseter motor function, receives glutamatergic inputs mainly from the parvocellular reticular formation (PCRt), but also from the adjacent paramedian reticular area (PMnR). On the other hand, the Mo5 and PCRt do not receive direct input from the sublaterodorsal (SLD) nucleus, a brainstem region critical for REMS atonia of postural muscles. We hypothesized that the PCRt-PMnR, but not the SLD, regulates masseter phasic activity during REMS.
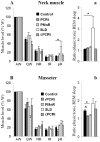
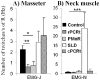
Methodology/principal findings: To test our hypothesis, we measured masseter electromyogram (EMG), neck muscle EMG, electrooculogram (EOG) and EEG in rats with cell-body specific lesions of the SLD, PMnR, and PCRt. Bilateral lesions of the PMnR and rostral PCRt (rPCRt), but not the caudal PCRt or SLD, reduced and eliminated REMS phasic activity of the masseter, respectively. Lesions of the PMnR and rPCRt did not, however, alter the neck EMG or EOG. To determine if rPCRt neurons use glutamate to control masseter phasic movements, we selectively blocked glutamate release by rPCRt neurons using a Cre-lox mouse system. Genetic disruption of glutamate neurotransmission by rPCRt neurons blocked masseter phasic activity during REMS.
Conclusions/significance: These results indicate that (1) premotor glutamatergic neurons in the medullary rPCRt and PMnR are involved in generating phasic activity in the masseter muscles, but not phasic eye movements, during REMS; and (2) separate brainstem neural circuits control postural and cranial muscle phasic activity during REMS.
Conflict of interest statement
Figures

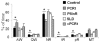


References
-
- Jouvet M, Michel F, Courjon J. [On a stage of rapid cerebral electrical activity in the course of physiological sleep.]. C R Seances Soc Biol Fil. 1959;153:1024–1028. - PubMed
-
- Jouvet M, Michel F. [Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat.]. C R Seances Soc Biol Fil. 1959;153:422–425. - PubMed
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Kato T, Masuda Y, Kanayama H, Morimoto T. Muscle activities are differently modulated between masseter and neck muscle during sleep-wake cycles in guinea pigs. Neurosci Res. 2007;58:265–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

