A single amino acid change in Ca(v)1.2 channels eliminates the permeation and gating differences between Ca(2+) and Ba(2+)
- PMID: 20098982
- PMCID: PMC3704197
- DOI: 10.1007/s00232-009-9221-1
A single amino acid change in Ca(v)1.2 channels eliminates the permeation and gating differences between Ca(2+) and Ba(2+)
Abstract
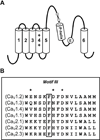
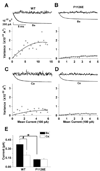
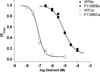
Glutamate scanning mutagenesis was used to assess the role of the calcicludine binding segment in regulating channel permeation and gating using both Ca(2+) and Ba(2+) as charge carriers. As expected, wild-type Ca(V)1.2 channels had a Ba(2+) conductance ~2x that in Ca(2+) (G(Ba)/G(Ca) = 2) and activation was ~10 mV more positive in Ca(2+) vs. Ba(2+). Of the 11 mutants tested, F1126E was the only one that showed unique permeation and gating properties compared to the wild type. F1126E equalized the Ca(V)1.2 channel conductance (G(Ba)/G(Ca) = 1) and activation voltage dependence between Ca(2+) and Ba(2+). Ba(2+) permeation was reduced because the interactions among multiple Ba(2+) ions and the pore were specifically altered for F1126E, which resulted in Ca(2+)-like ionic conductance and unitary current. However, the high-affinity block of monovalent cation flux was not altered for either Ca(2+) or Ba(2+). The half-activation voltage of F1126E in Ba(2+) was depolarized to match that in Ca(2+), which was unchanged from that in the wild type. As a result, the voltages for half-activation and half-inactivation of F1126E in Ba(2+) and Ca(2+) were similar to those of wild-type in Ca(2+). This effect was specific to F1126E since F1126A did not affect the half-activation voltage in either Ca(2+) or Ba(2+). These results indicate that residues in the outer vestibule of the Ca(V)1.2 channel pore are major determinants of channel gating, selectivity, and permeation.
Figures


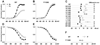


Similar articles
-
Amino acid substitutions in the pore of the Ca(V)1.2 calcium channel reduce barium currents without affecting calcium currents.Biophys J. 2005 Sep;89(3):1731-43. doi: 10.1529/biophysj.104.058875. Epub 2005 Jun 24. Biophys J. 2005. PMID: 15980164 Free PMC article.
-
Permeation and gating in CaV3.1 (alpha1G) T-type calcium channels effects of Ca2+, Ba2+, Mg2+, and Na+.J Gen Physiol. 2008 Aug;132(2):223-38. doi: 10.1085/jgp.200809986. J Gen Physiol. 2008. PMID: 18663131 Free PMC article.
-
Block of CaV1.2 channels by Gd3+ reveals preopening transitions in the selectivity filter.J Gen Physiol. 2007 Jun;129(6):461-75. doi: 10.1085/jgp.200709733. J Gen Physiol. 2007. PMID: 17535959 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
Cited by
-
CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries.J Gen Physiol. 2015 May;145(5):405-18. doi: 10.1085/jgp.201511361. J Gen Physiol. 2015. PMID: 25918359 Free PMC article.
-
T-type calcium channels and vascular function: the new kid on the block?J Physiol. 2011 Feb 15;589(Pt 4):783-95. doi: 10.1113/jphysiol.2010.199497. Epub 2010 Dec 20. J Physiol. 2011. PMID: 21173074 Free PMC article. Review.
-
Lead poisoning: acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels.Biophys Rev. 2017 Oct;9(5):807-825. doi: 10.1007/s12551-017-0303-5. Epub 2017 Aug 23. Biophys Rev. 2017. PMID: 28836190 Free PMC article. Review.
References
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

