A single amino acid change in Ca(v)1.2 channels eliminates the permeation and gating differences between Ca(2+) and Ba(2+)
- PMID: 20098982
- PMCID: PMC3704197
- DOI: 10.1007/s00232-009-9221-1
A single amino acid change in Ca(v)1.2 channels eliminates the permeation and gating differences between Ca(2+) and Ba(2+)
Abstract
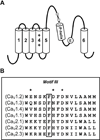
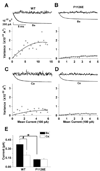
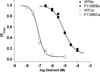
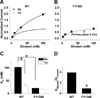
Glutamate scanning mutagenesis was used to assess the role of the calcicludine binding segment in regulating channel permeation and gating using both Ca(2+) and Ba(2+) as charge carriers. As expected, wild-type Ca(V)1.2 channels had a Ba(2+) conductance ~2x that in Ca(2+) (G(Ba)/G(Ca) = 2) and activation was ~10 mV more positive in Ca(2+) vs. Ba(2+). Of the 11 mutants tested, F1126E was the only one that showed unique permeation and gating properties compared to the wild type. F1126E equalized the Ca(V)1.2 channel conductance (G(Ba)/G(Ca) = 1) and activation voltage dependence between Ca(2+) and Ba(2+). Ba(2+) permeation was reduced because the interactions among multiple Ba(2+) ions and the pore were specifically altered for F1126E, which resulted in Ca(2+)-like ionic conductance and unitary current. However, the high-affinity block of monovalent cation flux was not altered for either Ca(2+) or Ba(2+). The half-activation voltage of F1126E in Ba(2+) was depolarized to match that in Ca(2+), which was unchanged from that in the wild type. As a result, the voltages for half-activation and half-inactivation of F1126E in Ba(2+) and Ca(2+) were similar to those of wild-type in Ca(2+). This effect was specific to F1126E since F1126A did not affect the half-activation voltage in either Ca(2+) or Ba(2+). These results indicate that residues in the outer vestibule of the Ca(V)1.2 channel pore are major determinants of channel gating, selectivity, and permeation.
Figures


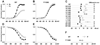


References
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

